Role of rhodopsin and arrestin phosphorylation in retinal degeneration of Drosophila
- PMID: 22855823
- PMCID: PMC3430379
- DOI: 10.1523/JNEUROSCI.0565-12.2012
Role of rhodopsin and arrestin phosphorylation in retinal degeneration of Drosophila
Abstract
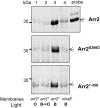
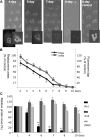
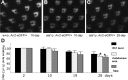
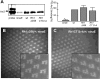
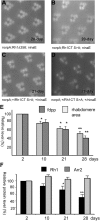
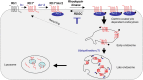
Arrestins belong to a family of multifunctional adaptor proteins that regulate internalization of diverse receptors including G-protein-coupled receptors (GPCRs). Defects associated with endocytosis of GPCRs have been linked to human diseases. We used enhanced green fluorescent protein-tagged arrestin 2 (Arr2) to monitor the turnover of the major rhodopsin (Rh1) in live Drosophila. We demonstrate that during degeneration of norpA(P24) photoreceptors the loss of Rh1 is parallel to the disappearance of rhabdomeres, the specialized visual organelle that houses Rh1. The cause of degeneration in norpA(P24) is the failure to activate CaMKII (Ca(2+)/calmodulin-dependent protein kinase II) and retinal degeneration C (RDGC) because of a loss of light-dependent Ca(2+) entry. A lack of activation in CaMKII, which phosphorylates Arr2, leads to hypophosphorylated Arr2, while a lack of activation of RDGC, which dephosphorylates Rh1, results in hyperphosphorylated Rh1. We investigated how reversible phosphorylation of Rh1 and Arr2 contributes to photoreceptor degeneration. To uncover the consequence underlying a lack of CaMKII activation, we characterized ala(1) flies in which CaMKII was suppressed by an inhibitory peptide, and showed that morphology of rhabdomeres was not affected. In contrast, we found that expression of phosphorylation-deficient Rh1s, which either lack the C terminus or contain Ala substitution in the phosphorylation sites, was able to prevent degeneration of norpA(P24) photoreceptors. This suppression is not due to a loss of Arr2 interaction. Importantly, co-expression of these modified Rh1s offered protective effects, which greatly delayed photoreceptor degeneration. Together, we conclude that phosphorylation of Rh1 is the major determinant that orchestrates its internalization leading to retinal degeneration.
Figures

Similar articles
-
Exploring Excitotoxicity and Regulation of a Constitutively Active TRP Ca2+ Channel in Drosophila.Fly (Austin). 2021 Dec;15(1):8-27. doi: 10.1080/19336934.2020.1851586. Epub 2020 Dec 1. Fly (Austin). 2021. PMID: 33200658 Free PMC article.
-
Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival.Curr Biol. 2005 Oct 11;15(19):1722-33. doi: 10.1016/j.cub.2005.08.064. Curr Biol. 2005. PMID: 16213818
-
Drosophila king tubby (ktub) mediates light-induced rhodopsin endocytosis and retinal degeneration.J Biomed Sci. 2012 Dec 10;19(1):101. doi: 10.1186/1423-0127-19-101. J Biomed Sci. 2012. PMID: 23228091 Free PMC article.
-
Molecular genetics of retinal degeneration: A Drosophila perspective.Fly (Austin). 2011 Oct-Dec;5(4):356-68. doi: 10.4161/fly.5.4.17809. Epub 2011 Sep 7. Fly (Austin). 2011. PMID: 21897116 Free PMC article. Review.
-
Rhodopsin mutations as the cause of retinal degeneration. Classification of degeneration phenotypes in the model system Drosophila melanogaster.Acta Anat (Basel). 1998;162(2-3):85-94. doi: 10.1159/000046472. Acta Anat (Basel). 1998. PMID: 9831754 Review.
Cited by
-
Exploring Excitotoxicity and Regulation of a Constitutively Active TRP Ca2+ Channel in Drosophila.Fly (Austin). 2021 Dec;15(1):8-27. doi: 10.1080/19336934.2020.1851586. Epub 2020 Dec 1. Fly (Austin). 2021. PMID: 33200658 Free PMC article.
-
Protein Phosphatase 2A and Clathrin-Mediated Endocytosis Facilitate Robust Melanopsin Light Responses and Resensitization.Invest Ophthalmol Vis Sci. 2020 Oct 1;61(12):10. doi: 10.1167/iovs.61.12.10. Invest Ophthalmol Vis Sci. 2020. PMID: 33049058 Free PMC article.
-
Arrestins in apoptosis.Handb Exp Pharmacol. 2014;219:309-39. doi: 10.1007/978-3-642-41199-1_16. Handb Exp Pharmacol. 2014. PMID: 24292837 Free PMC article.
-
Distinct roles of arrestin 1 protein in photoreceptors during Drosophila development.J Biol Chem. 2014 Jun 27;289(26):18526-34. doi: 10.1074/jbc.M114.571224. Epub 2014 May 16. J Biol Chem. 2014. PMID: 24838243 Free PMC article.
-
The Role of Reversible Phosphorylation of Drosophila Rhodopsin.Int J Mol Sci. 2022 Nov 24;23(23):14674. doi: 10.3390/ijms232314674. Int J Mol Sci. 2022. PMID: 36499010 Free PMC article. Review.
References
-
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
