Cryo-electron tomography of rubella virus
- PMID: 22855483
- PMCID: PMC3457135
- DOI: 10.1128/JVI.01390-12
Cryo-electron tomography of rubella virus
Abstract
Rubella virus is the only member of the Rubivirus genus within the Togaviridae family and is the causative agent of the childhood disease known as rubella or German measles. Here, we report the use of cryo-electron tomography to examine the three-dimensional structure of rubella virions and compare their structure to that of Ross River virus, a togavirus belonging the genus Alphavirus. The ectodomains of the rubella virus glycoproteins, E1 and E2, are shown to be organized into extended rows of density, separated by 9 nm on the viral surface. We also show that the rubella virus nucleocapsid structure often forms a roughly spherical shell which lacks high density at its center. While many rubella virions are approximately spherical and have dimensions similar to that of the icosahedral Ross River virus, the present results indicate that rubella exhibits a large degree of pleomorphy. In addition, we used rotation function calculations and other analyses to show that approximately spherical rubella virions lack the icosahedral organization which characterizes Ross River and other alphaviruses. The present results indicate that the assembly mechanism of rubella virus, which has previously been shown to differ from that of the alphavirus assembly pathway, leads to an organization of the rubella virus structural proteins that is different from that of alphaviruses.
Figures
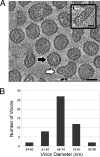
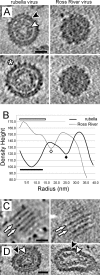
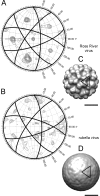
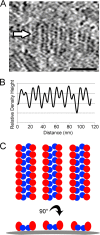
Similar articles
-
Assembly, maturation and three-dimensional helical structure of the teratogenic rubella virus.PLoS Pathog. 2017 Jun 2;13(6):e1006377. doi: 10.1371/journal.ppat.1006377. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28575072 Free PMC article.
-
Nucleocapsid and glycoprotein organization in an enveloped virus.Cell. 1995 Feb 24;80(4):621-30. doi: 10.1016/0092-8674(95)90516-2. Cell. 1995. PMID: 7867069 Free PMC article.
-
Nucleocapsid-glycoprotein interactions required for assembly of alphaviruses.J Virol. 1994 Mar;68(3):1316-23. doi: 10.1128/JVI.68.3.1316-1323.1994. J Virol. 1994. PMID: 7508993 Free PMC article.
-
[Virology of the family Togaviridae].Uirusu. 2011 Dec;61(2):211-9. doi: 10.2222/jsv.61.211. Uirusu. 2011. PMID: 22916568 Review. Japanese.
-
[The life cycle of Rubella Virus].Uirusu. 2014;64(2):137-46. doi: 10.2222/jsv.64.137. Uirusu. 2014. PMID: 26437836 Review. Japanese.
Cited by
-
The Density Code for the Development of a Vaccine?J Pharm Sci. 2016 Nov;105(11):3223-3232. doi: 10.1016/j.xphs.2016.07.020. Epub 2016 Sep 17. J Pharm Sci. 2016. PMID: 27649885 Free PMC article. Review.
-
Rubella.Lancet. 2015 Jun 6;385(9984):2297-307. doi: 10.1016/S0140-6736(14)60539-0. Epub 2015 Jan 8. Lancet. 2015. PMID: 25576992 Free PMC article. Review.
-
Rubella Virus Infections: A Bibliometric Analysis of the Scientific Literature from 2000 to 2021.Healthcare (Basel). 2022 Dec 17;10(12):2562. doi: 10.3390/healthcare10122562. Healthcare (Basel). 2022. PMID: 36554085 Free PMC article.
-
Study protocol for a phase 1/2, single-centre, double-blind, double-dummy, randomized, active-controlled, age de-escalation trial to assess the safety, tolerability and immunogenicity of a measles and rubella vaccine delivered by a microneedle patch in healthy adults (18 to 40 years), measles and rubella vaccine-primed toddlers (15 to 18 months) and measles and rubella vaccine-naïve infants (9 to 10 months) in The Gambia [Measles and Rubella Vaccine Microneedle Patch Phase 1/2 Age De-escalation Trial].Trials. 2022 Sep 14;23(1):775. doi: 10.1186/s13063-022-06493-5. Trials. 2022. PMID: 36104719 Free PMC article.
-
Exogenous Rubella Virus Capsid Proteins Enhance Virus Genome Replication.Pathogens. 2022 Jun 14;11(6):683. doi: 10.3390/pathogens11060683. Pathogens. 2022. PMID: 35745537 Free PMC article.
References
-
- Agarwal RC. 1978. A new least-squares refinement technique based on the fast Fourier transform algorithm. Acta Crystallogr. Sect. A 34:791–809
-
- Aliperti G, Schlesinger MJ. 1978. Evidence for an autoprotease activity of Sindbis virus capsid protein. Virology 90:366–369 - PubMed
-
- Bardeletti G, Gautheron DC. 1976. Phospholipid and cholesterol composition of rubella virus and its host cell BHK 21 grown in suspension cultures. Arch. Virol. 52:19–27 - PubMed
-
- Bardeletti G, Tektoff J, Gautheron D. 1979. Rubella virus maturation and production in two host cell systems. Intervirology 11:97–103 - PubMed
-
- Baron MD, Forsell K. 1991. Oligomerization of the structural proteins of rubella virus. Virology 185:811–819 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

