Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death
- PMID: 22854956
- PMCID: PMC3442531
- DOI: 10.1074/jbc.M112.355172
Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death
Abstract
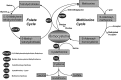
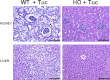
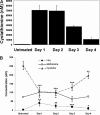
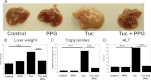
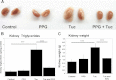
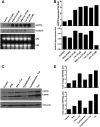
Cystathionine (R-S-(2-amino-2-carboxyethyl)-l-homocysteine) is a non-proteinogenic thioether containing amino acid. In mammals, cystathionine is formed as an intermediate of the transsulfuration pathway by the condensation of serine and homocysteine (Hcy) in a reaction catalyzed by cystathionine β-synthase (CBS). Cystathionine is subsequently converted to cysteine plus ammonia and α-ketobutyrate by the action of cystathionine γ-lyase (CGL). Pathogenic mutations in CBS result in CBS-deficient homocystinuria (HCU) which, if untreated, results in mental retardation, thromboembolic complications and connective tissue disorders. Currently there is no known function for cystathionine other than serving as an intermediate in transsulfuration and to date, the possible contribution of the abolition of cystathionine synthesis to pathogenesis in HCU has not been investigated. Using both mouse and cell-culture models, we have found that cystathionine is capable of blocking the induction of hepatic steatosis and kidney injury, acute tubular necrosis, and apoptotic cell death by the endoplasmic reticulum stress inducing agent tunicamycin. Northern and Western blotting analysis indicate that the protective effects of cystathionine occur without any obvious alteration of the induction of the unfolded protein response. Our data constitute the first experimental evidence that the abolition of cystathionine synthesis may contribute to the pathology of HCU and that this compound has therapeutic potential for disease states where ER stress is implicated as a primary initiating pathogenic factor.
Figures



Similar articles
-
Characterization of two pathogenic mutations in cystathionine beta-synthase: different intracellular locations for wild-type and mutant proteins.Gene. 2013 Nov 15;531(1):117-24. doi: 10.1016/j.gene.2013.08.021. Epub 2013 Aug 24. Gene. 2013. PMID: 23981774 Free PMC article.
-
A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment.Mol Genet Metab. 2010 Oct-Nov;101(2-3):153-62. doi: 10.1016/j.ymgme.2010.06.010. Epub 2010 Jun 23. Mol Genet Metab. 2010. PMID: 20638879 Free PMC article.
-
Derangement of hepatic polyamine, folate, and methionine cycle metabolism in cystathionine beta-synthase-deficient homocystinuria in the presence and absence of treatment: Possible implications for pathogenesis.Mol Genet Metab. 2021 Feb;132(2):128-138. doi: 10.1016/j.ymgme.2021.01.003. Epub 2021 Jan 11. Mol Genet Metab. 2021. PMID: 33483253
-
Classical homocystinuria: From cystathionine beta-synthase deficiency to novel enzyme therapies.Biochimie. 2020 Jun;173:48-56. doi: 10.1016/j.biochi.2019.12.007. Epub 2019 Dec 16. Biochimie. 2020. PMID: 31857119 Review.
-
Recent therapeutic approaches to cystathionine beta-synthase-deficient homocystinuria.Br J Pharmacol. 2023 Feb;180(3):264-278. doi: 10.1111/bph.15991. Epub 2022 Dec 8. Br J Pharmacol. 2023. PMID: 36417581 Free PMC article. Review.
Cited by
-
Enzyme replacement with PEGylated cystathionine β-synthase ameliorates homocystinuria in murine model.J Clin Invest. 2016 Jun 1;126(6):2372-84. doi: 10.1172/JCI85396. Epub 2016 May 16. J Clin Invest. 2016. PMID: 27183385 Free PMC article.
-
Analysis of differential neonatal lethality in cystathionine β-synthase deficient mouse models using metabolic profiling.FASEB J. 2021 Jun;35(6):e21629. doi: 10.1096/fj.202100302R. FASEB J. 2021. PMID: 33949005 Free PMC article.
-
L-Cystathionine Protects against Homocysteine-Induced Mitochondria-Dependent Apoptosis of Vascular Endothelial Cells.Oxid Med Cell Longev. 2019 Nov 25;2019:1253289. doi: 10.1155/2019/1253289. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885769 Free PMC article.
-
Endoplasmic Reticulum Stress and Autophagy in Homocystinuria Patients with Remethylation Defects.PLoS One. 2016 Mar 9;11(3):e0150357. doi: 10.1371/journal.pone.0150357. eCollection 2016. PLoS One. 2016. PMID: 26959487 Free PMC article.
-
Role of Cystathionine Gamma-Lyase in Immediate Renal Impairment and Inflammatory Response in Acute Ischemic Kidney Injury.Sci Rep. 2016 Jun 8;6:27517. doi: 10.1038/srep27517. Sci Rep. 2016. PMID: 27273292 Free PMC article.
References
-
- Mudd S. H., Levy H. L., Kraus J. P. (2001) in The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K., Vogelstein B., eds), pp. 2007–2056, 8 Ed., McGraw-Hill, New York
-
- Maclean K. N., Sikora J., Kožich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Kraus J. P. (2010) Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol. Genet. Metab. 101, 163–171 - PMC - PubMed
-
- Maclean K. N., Sikora J., Kožich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Overdier K. H., Collard R., Brodsky G. L., Meltesen L., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Rozen R., Patterson D., Kraus J. P. (2010) A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol. Genet. Metab. 101, 153–162 - PMC - PubMed
-
- Werstuck G. H., Lentz S. R., Dayal S., Hossain G. S., Sood S. K., Shi Y. Y., Zhou J., Maeda N., Krisans S. K., Malinow M. R., Austin R. C. (2001) Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Invest. 107, 1263–1273 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

