Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins
- PMID: 22850678
- PMCID: PMC3493462
- DOI: 10.1038/mt.2012.144
Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins
Abstract
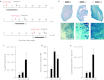
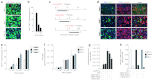
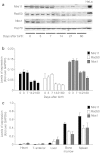
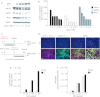
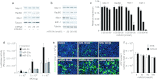
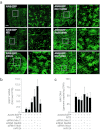
Gene therapy vectors based on the adeno-associated virus (AAV) are extremely efficient for gene transfer into post-mitotic cells of heart, muscle, brain, and retina. The reason for their exquisite tropism for these cells has long remained elusive. Here, we show that upon terminal differentiation, cardiac and skeletal myocytes downregulate proteins of the DNA damage response (DDR) and that this markedly induces permissivity to AAV transduction. We observed that expression of members of the MRN complex (Mre11, Rad50, Nbs1), which bind the incoming AAV genomes, faded in cardiomyocytes at ~2 weeks after birth, as well as upon myoblast differentiation in vitro; in both cases, withdrawal of the cells from the cell cycle coincided with increased AAV permissivity. Treatment of proliferating cells with short-interfering RNAs (siRNAs) against the MRN proteins, or with microRNA-24, which is normally upregulated upon terminal differentiation and negatively controls the Nbs1 levels, significantly increased permissivity to AAV transduction. Consistently, delivery of these small RNAs to the juvenile liver concomitant with AAV markedly improved in vivo hepatocyte transduction. Collectively, these findings support the conclusion that cellular DDR proteins inhibit AAV transduction and that terminal cell differentiation relieves this restriction.
Figures

Similar articles
-
Insight into the mechanism of inhibition of adeno-associated virus by the Mre11/Rad50/Nbs1 complex.J Virol. 2015 Jan;89(1):181-94. doi: 10.1128/JVI.01990-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320294 Free PMC article.
-
Impact of the MRN Complex on Adeno-Associated Virus Integration and Replication during Coinfection with Herpes Simplex Virus 1.J Virol. 2015 Jul;89(13):6824-34. doi: 10.1128/JVI.00171-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903339 Free PMC article.
-
Processing of recombinant AAV genomes occurs in specific nuclear structures that overlap with foci of DNA-damage-response proteins.J Cell Sci. 2008 Feb 1;121(Pt 3):349-57. doi: 10.1242/jcs.003632. J Cell Sci. 2008. PMID: 18216333
-
Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template.Biochem Cell Biol. 2007 Aug;85(4):509-20. doi: 10.1139/O07-069. Biochem Cell Biol. 2007. PMID: 17713585 Review.
-
MRN (MRE11-RAD50-NBS1) Complex in Human Cancer and Prognostic Implications in Colorectal Cancer.Int J Mol Sci. 2019 Feb 14;20(4):816. doi: 10.3390/ijms20040816. Int J Mol Sci. 2019. PMID: 30769804 Free PMC article. Review.
Cited by
-
Adeno-associated Virus Vectors Efficiently Transduce Mouse and Rabbit Sensory Neurons Coinfected with Herpes Simplex Virus 1 following Peripheral Inoculation.J Virol. 2016 Aug 12;90(17):7894-901. doi: 10.1128/JVI.01028-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334582 Free PMC article.
-
Gene Therapy With Angiotensin-(1-9) Preserves Left Ventricular Systolic Function After Myocardial Infarction.J Am Coll Cardiol. 2016 Dec 20;68(24):2652-2666. doi: 10.1016/j.jacc.2016.09.946. J Am Coll Cardiol. 2016. PMID: 27978950 Free PMC article.
-
Gene therapy in monogenic congenital myopathies.Methods. 2016 Apr 15;99:91-8. doi: 10.1016/j.ymeth.2015.10.004. Epub 2015 Oct 14. Methods. 2016. PMID: 26454198 Free PMC article. Review.
-
Interactions between miRNAs and Double-Strand Breaks DNA Repair Genes, Pursuing a Fine-Tuning of Repair.Int J Mol Sci. 2022 Mar 17;23(6):3231. doi: 10.3390/ijms23063231. Int J Mol Sci. 2022. PMID: 35328651 Free PMC article. Review.
-
Depletion of the Insulator Protein CTCF Results in Herpes Simplex Virus 1 Reactivation In Vivo.J Virol. 2018 May 14;92(11):e00173-18. doi: 10.1128/JVI.00173-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514910 Free PMC article.
References
-
- Mingozzi F., and, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. - PubMed
-
- Wu Z, Asokan A., and, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. - PubMed
-
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P., and, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

