Mapping MKP-3/FOXO1 interaction and evaluating the effect on gluconeogenesis
- PMID: 22848439
- PMCID: PMC3405053
- DOI: 10.1371/journal.pone.0041168
Mapping MKP-3/FOXO1 interaction and evaluating the effect on gluconeogenesis
Abstract
Background: MAP kinase phosphatase 3 (MKP-3) is known to attenuate the ERK signaling pathway. It has been recently demonstrated that MKP-3 is also a player in promoting hepatic glucose output in obese state by interacting and activating FOXO1. Reduction of hepatic MKP-3 expression is sufficient to reduce blood glucose levels in both diet-induced and genetically obese mice.
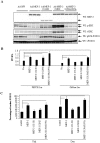
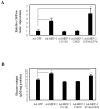
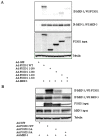
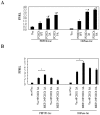
Methodology/principal findings: In current study, the mechanism of MKP-3/FOXO1 interaction and the effects on transcription of gluconeogenic gene and glucose output was investigated in Fao hepatoma cells by using mutated MKP-3 and FOXO1 adenoviral constructs. The results indicate that MKP-3 phosphatase activity is not required for MKP-3/FOXO1 interaction but is essential for FOXO1 nuclear translocation and MKP-3 promoted gluconeogenesis. Compared to GFP control (1±0.38), MKP-3 increased G6Pase gene expression by 242% (3.42±0.62) while inactive MKP-3 does not change G6Pase expression (0.98±0.17). The residues 200-260 of MKP-3 and the residues 360-456 of FOXO1 are essential for mediating MKP-3/FOXO1 interaction. Interestingly, ERK phosphorylation deficient but not Akt phosphorylation deficient FOXO1 mutant lost interaction with MKP-3. Furthermore, in vivo experiments showed that Akt phosphorylation resistant FOXO1 3A mutant is sufficient to rescue the hypoglycemia caused by MKP-3 knock down in the liver of lean mice (from 141±6.78 to 209±14.64 mg/dL).
Conclusions/significance: 1) Critical residues mediating MKP-3/FOXO1 interaction have been identified; 2) ERK phosphorylation deficient FOXO1 mutant is as potent as Akt phosphorylation deficient FOXO1 mutant in activating transcription of gluconeogenic genes; 3) Constitutively active FOXO1 can rescue the hypoglycemic effect caused by reduced hepatic MKP-3 expression in vivo.
Conflict of interest statement
Figures

Similar articles
-
FOXO1-dependent up-regulation of MAP kinase phosphatase 3 (MKP-3) mediates glucocorticoid-induced hepatic lipid accumulation in mice.Mol Cell Endocrinol. 2014 Aug 5;393(1-2):46-55. doi: 10.1016/j.mce.2014.06.001. Epub 2014 Jun 16. Mol Cell Endocrinol. 2014. PMID: 24946098 Free PMC article.
-
Exercise training decreases mitogen-activated protein kinase phosphatase-3 expression and suppresses hepatic gluconeogenesis in obese mice.J Physiol. 2014 Mar 15;592(6):1325-40. doi: 10.1113/jphysiol.2013.264002. Epub 2014 Jan 6. J Physiol. 2014. PMID: 24396063 Free PMC article.
-
MAPK phosphatase-3 promotes hepatic gluconeogenesis through dephosphorylation of forkhead box O1 in mice.J Clin Invest. 2010 Nov;120(11):3901-11. doi: 10.1172/JCI43250. J Clin Invest. 2010. PMID: 20921625 Free PMC article.
-
Inhibition of gluconeogenesis in primary hepatocytes by stromal cell-derived factor-1 (SDF-1) through a c-Src/Akt-dependent signaling pathway.J Biol Chem. 2008 Nov 7;283(45):30642-9. doi: 10.1074/jbc.M803698200. Epub 2008 Sep 11. J Biol Chem. 2008. PMID: 18786922 Free PMC article.
-
CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis.BMB Rep. 2013 Dec;46(12):567-74. doi: 10.5483/bmbrep.2013.46.12.248. BMB Rep. 2013. PMID: 24238363 Free PMC article. Review.
Cited by
-
Dual-specificity MAP kinase phosphatases in health and disease.Biochim Biophys Acta Mol Cell Res. 2019 Jan;1866(1):124-143. doi: 10.1016/j.bbamcr.2018.09.002. Epub 2018 Sep 8. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30401534 Free PMC article. Review.
-
Dual specificity phosphatase 6 deficiency is associated with impaired systemic glucose tolerance and reversible weight retardation in mice.PLoS One. 2017 Sep 5;12(9):e0183488. doi: 10.1371/journal.pone.0183488. eCollection 2017. PLoS One. 2017. PMID: 28873424 Free PMC article.
-
Systems analysis of drug-induced receptor tyrosine kinase reprogramming following targeted mono- and combination anti-cancer therapy.Cells. 2014 Jun 10;3(2):563-91. doi: 10.3390/cells3020563. Cells. 2014. PMID: 24918976 Free PMC article.
-
Mitogen-Activated Protein Kinase Regulation in Hepatic Metabolism.Trends Endocrinol Metab. 2017 Dec;28(12):868-878. doi: 10.1016/j.tem.2017.10.007. Epub 2017 Nov 8. Trends Endocrinol Metab. 2017. PMID: 29128158 Free PMC article. Review.
-
FOXO1-dependent up-regulation of MAP kinase phosphatase 3 (MKP-3) mediates glucocorticoid-induced hepatic lipid accumulation in mice.Mol Cell Endocrinol. 2014 Aug 5;393(1-2):46-55. doi: 10.1016/j.mce.2014.06.001. Epub 2014 Jun 16. Mol Cell Endocrinol. 2014. PMID: 24946098 Free PMC article.
References
-
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. - PubMed
-
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. - PubMed
-
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. - PubMed
-
- Myers MG, Jr, White MF. Insulin signal transduction and the IRS proteins. Annu Rev Pharmacol Toxicol. 1996;36:615–658. - PubMed
-
- Holman GD, Kasuga M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia. 1997;40:991–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

