Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma
- PMID: 22847020
- PMCID: PMC3541928
- DOI: 10.1007/s00262-012-1319-0
Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma
Abstract
Background: This study evaluated the safety and immune responses to an autologous dendritic cell vaccine pulsed with class I peptides from tumor-associated antigens (TAA) expressed on gliomas and overexpressed in their cancer stem cell population (ICT-107).
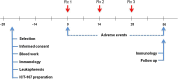
Methods: TAA epitopes included HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2. HLA-A1- and/or HLA-A2-positive patients with glioblastoma (GBM) were eligible. Mononuclear cells from leukapheresis were differentiated into dendritic cells, pulsed with TAA peptides, and administered intradermally three times at two-week intervals.
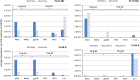
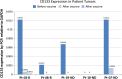
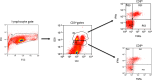
Results: Twenty-one patients were enrolled with 17 newly diagnosed (ND-GBM) and three recurrent GBM patients and one brainstem glioma. Immune response data on 15 newly diagnosed patients showed 33 % responders. TAA expression by qRT-PCR from fresh-frozen tumor samples showed all patient tumors expressed at least three TAA, with 75 % expressing all six. Correlations of increased PFS and OS with quantitative expression of MAGE1 and AIM-2 were observed, and a trend for longer survival was observed with gp100 and HER2 antigens. Target antigens gp100, HER1, and IL13Rα2 were downregulated in recurrent tumors from 4 HLA-A2+ patients. A decrease in or absence of CD133 expression was seen in five patients who underwent a second resection. At a median follow-up of 40.1 months, six of 16 ND-GBM patients showed no evidence of tumor recurrence. Median PFS in newly diagnosed patients was 16.9 months, and median OS was 38.4 months.
Conclusions: Expression of four ICT-107 targeted antigens in the pre-vaccine tumors correlated with prolonged overall survival and PFS in ND-GBM patients. The goal of targeting tumor antigens highly expressed on glioblastoma cancer stem cells is supported by the observation of decreased or absent CD133 expression in the recurrent areas of gadolinium-enhanced tumors.
Conflict of interest statement
JSY and JGB were salaried employees of and owned stock in ImmunoCellular Therapeutics. ESH and KLB were consultants for and owned stock in ImmunoCellular Therapeutics. All other authors declare that they have no conflicts of interest.
Figures


Similar articles
-
A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma.Clin Cancer Res. 2019 Oct 1;25(19):5799-5807. doi: 10.1158/1078-0432.CCR-19-0261. Epub 2019 Jul 18. Clin Cancer Res. 2019. PMID: 31320597 Free PMC article. Clinical Trial.
-
Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report.Neurosurg Focus. 2000 Dec 15;9(6):e8. doi: 10.3171/foc.2000.9.6.9. Neurosurg Focus. 2000. PMID: 16817691
-
α-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial.BMC Cancer. 2012 Dec 27;12:623. doi: 10.1186/1471-2407-12-623. BMC Cancer. 2012. PMID: 23270484 Free PMC article. Clinical Trial.
-
Current vaccine trials in glioblastoma: a review.J Immunol Res. 2014;2014:796856. doi: 10.1155/2014/796856. Epub 2014 Apr 3. J Immunol Res. 2014. PMID: 24804271 Free PMC article. Review.
-
DCVax®-L--developed by Northwest Biotherapeutics.Hum Vaccin Immunother. 2014;10(11):3139-45. doi: 10.4161/hv.29276. Hum Vaccin Immunother. 2014. PMID: 25483653 Free PMC article. Review.
Cited by
-
Personalized medicine for gliomas.Surg Neurol Int. 2015 Feb 13;6(Suppl 1):S89-95. doi: 10.4103/2152-7806.151351. eCollection 2015. Surg Neurol Int. 2015. PMID: 25722938 Free PMC article.
-
Development of dendritic cell loaded MAGE-A2 long peptide; a potential target for tumor-specific T cell-mediated prostate cancer immunotherapy.Cancer Cell Int. 2023 Nov 11;23(1):270. doi: 10.1186/s12935-023-03108-0. Cancer Cell Int. 2023. PMID: 37951911 Free PMC article.
-
Genetics and immunotherapy: using the genetic landscape of gliomas to inform management strategies.J Neurooncol. 2015 Jul;123(3):373-83. doi: 10.1007/s11060-015-1730-4. Epub 2015 Feb 21. J Neurooncol. 2015. PMID: 25697584 Review.
-
CD133 Expression Is Not Synonymous to Immunoreactivity for AC133 and Fluctuates throughout the Cell Cycle in Glioma Stem-Like Cells.PLoS One. 2015 Jun 18;10(6):e0130519. doi: 10.1371/journal.pone.0130519. eCollection 2015. PLoS One. 2015. PMID: 26086074 Free PMC article.
-
Myeloidcells in the immunosuppressive microenvironment in glioblastoma: The characteristics and therapeutic strategies.Front Immunol. 2023 Feb 27;14:994698. doi: 10.3389/fimmu.2023.994698. eCollection 2023. Front Immunol. 2023. PMID: 36923402 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

