Aurora kinase A drives MTOC biogenesis but does not trigger resumption of meiosis in mouse oocytes matured in vivo
- PMID: 22837479
- PMCID: PMC3507544
- DOI: 10.1095/biolreprod.112.101014
Aurora kinase A drives MTOC biogenesis but does not trigger resumption of meiosis in mouse oocytes matured in vivo
Abstract
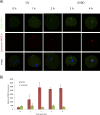
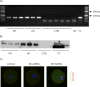
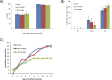
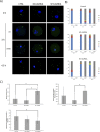
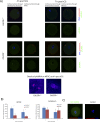
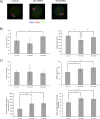
Aurora kinase A (AURKA) is an important mitotic kinase involved in the G2/M transition, centrosome maturation and separation, and spindle formation in somatic cells. We used transgenic models that specifically overexpress in mouse oocytes either wild-type (WT-AURKA) or a catalytically inactive (kinase-dead) (KD-AURKA) AURKA to gain new insights regarding the role of AURKA during oocyte maturation. AURKA activation occurs shortly after hCG administration that initiates maturation in vivo. Although AURKA activity is increased in WT-AURKA oocytes, resumption of meiosis is not observed in the absence of hCG administration. Control oocytes contain one to three microtubule organizing centers (MTOCs; centrosome equivalent) at prophase I. At the time of germinal vesicle breakdown (GVBD), the first visible marker of resumption of meiosis, the MTOC number increases. In WT-AURKA oocytes, the increase in MTOC number occurs prematurely but transiently without GVBD, whereas the increase in MTOC number does not occur in control and KD-AURKA oocytes. AURKA activation is biphasic with the initial activation not requiring CDC25B-CDK1 activity, whereas full activation, which is essential for the increase in MTOCs number, depends on CDK1 activity. AURKA activity also influences spindle length and regulates, independent of its protein kinase activity, the amount of MTOC associated with gamma-tubulin. Both WT-AURKA and KD-AURKA transgenic mice have normal fertility during first 6 mo of life. These results suggest that although AURKA is not a trigger kinase for G2/M transition in mouse oocytes, it regulates MTOC number and spindle length, and, independent of its protein kinase activity, gamma-tubulin recruitment to MTOCs.
Figures
Similar articles
-
Aurora kinase A controls meiosis I progression in mouse oocytes.Cell Cycle. 2008 Aug;7(15):2368-76. doi: 10.4161/cc.6361. Epub 2008 May 29. Cell Cycle. 2008. PMID: 18677115 Free PMC article.
-
Aurora kinase-A regulates microtubule organizing center (MTOC) localization, chromosome dynamics, and histone-H3 phosphorylation in mouse oocytes.Mol Reprod Dev. 2011 Feb;78(2):80-90. doi: 10.1002/mrd.21272. Epub 2011 Jan 27. Mol Reprod Dev. 2011. PMID: 21274965
-
Successive recruitment of p-CDC25B-Ser351 and p-cyclin B1-Ser123 to centrosomes contributes to the release of mouse oocytes from prophase I arrest.Dev Dyn. 2015 Feb;244(2):110-21. doi: 10.1002/dvdy.24220. Epub 2014 Nov 12. Dev Dyn. 2015. PMID: 25349079
-
Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells.Mol Hum Reprod. 2010 Sep;16(9):654-64. doi: 10.1093/molehr/gaq034. Epub 2010 May 7. Mol Hum Reprod. 2010. PMID: 20453035 Free PMC article. Review.
-
[Molecular Mechanism of Aurora Kinase A Regulating the Meiosis of Oocyte].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022 Feb;44(1):142-148. doi: 10.3881/j.issn.1000-503X.13811. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022. PMID: 35300777 Review. Chinese.
Cited by
-
Single human oocyte transcriptome analysis reveals distinct maturation stage-dependent pathways impacted by age.Aging Cell. 2021 May;20(5):e13360. doi: 10.1111/acel.13360. Epub 2021 Apr 28. Aging Cell. 2021. PMID: 33908703 Free PMC article.
-
RanGTP and importin β regulate meiosis I spindle assembly and function in mouse oocytes.EMBO J. 2020 Jan 2;39(1):e101689. doi: 10.15252/embj.2019101689. Epub 2019 Oct 16. EMBO J. 2020. PMID: 31617608 Free PMC article.
-
The chromosomal basis of meiotic acentrosomal spindle assembly and function in oocytes.Chromosoma. 2017 Jun;126(3):351-364. doi: 10.1007/s00412-016-0618-1. Epub 2016 Nov 11. Chromosoma. 2017. PMID: 27837282 Free PMC article. Review.
-
Mcrs1 regulates G2/M transition and spindle assembly during mouse oocyte meiosis.EMBO Rep. 2023 May 4;24(5):e56273. doi: 10.15252/embr.202256273. Epub 2023 Mar 23. EMBO Rep. 2023. PMID: 36951681 Free PMC article.
-
Predicting Infertility: How Genetic Variants in Oocyte Spindle Genes Affect Egg Quality.Adv Anat Embryol Cell Biol. 2024;238:1-22. doi: 10.1007/978-3-031-55163-5_1. Adv Anat Embryol Cell Biol. 2024. PMID: 39030352 Review.
References
-
- De Luca M, Lavia P, Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle 2006;5:296 303 - PubMed
-
- Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 2003;114:585 598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

