Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery
- PMID: 22826857
- PMCID: PMC4000586
- DOI: 10.4155/tde.11.14
Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery
Abstract
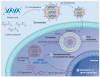
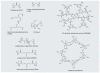
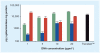
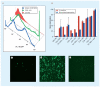
Nonviral systems for nucleic acid delivery offer a host of potential advantages compared with viruses, including reduced toxicity and immunogenicity, increased ease of production and less stringent vector size limitations, but remain far less efficient than their viral counterparts. In this article we review recent advances in the delivery of nucleic acids using polymeric and inorganic vectors. We discuss the wide range of materials being designed and evaluated for these purposes while considering the physical requirements and barriers to entry that these agents face and reviewing recent novel approaches towards improving delivery with respect to each of these barriers. Furthermore, we provide a brief overview of past and ongoing nonviral gene therapy clinical trials. We conclude with a discussion of multifunctional nucleic acid carriers and future directions.
Figures



Similar articles
-
Recent advances in nonviral vectors for gene delivery.Acc Chem Res. 2012 Jul 17;45(7):971-9. doi: 10.1021/ar200151m. Epub 2011 Aug 26. Acc Chem Res. 2012. PMID: 21870813 Free PMC article.
-
Nonviral approaches for neuronal delivery of nucleic acids.Pharm Res. 2008 May;25(5):983-98. doi: 10.1007/s11095-007-9439-5. Epub 2007 Oct 12. Pharm Res. 2008. PMID: 17932730 Free PMC article. Review.
-
Polymeric vectors for ocular gene delivery.Ther Deliv. 2011 Apr;2(4):523-36. doi: 10.4155/tde.11.20. Ther Deliv. 2011. PMID: 21858246 Free PMC article. Review.
-
Exploring the role of polymer structure on intracellular nucleic acid delivery via polymeric nanoparticles.J Control Release. 2015 Dec 10;219:488-499. doi: 10.1016/j.jconrel.2015.09.046. Epub 2015 Oct 1. J Control Release. 2015. PMID: 26433125 Free PMC article. Review.
-
Recent advances in characterization of nonviral vectors for delivery of nucleic acids: impact on their biological performance.Expert Opin Drug Deliv. 2015 Jan;12(1):27-39. doi: 10.1517/17425247.2014.945421. Epub 2014 Aug 21. Expert Opin Drug Deliv. 2015. PMID: 25141765 Review.
Cited by
-
Efficiency of Cytosolic Delivery with Poly(β-amino ester) Nanoparticles is Dependent on the Effective pKa of the Polymer.ACS Biomater Sci Eng. 2020 Jun 8;6(6):3411-3421. doi: 10.1021/acsbiomaterials.0c00271. Epub 2020 May 18. ACS Biomater Sci Eng. 2020. PMID: 33463158 Free PMC article.
-
2011 Rita Schaffer lecture: nanoparticles for intracellular nucleic acid delivery.Ann Biomed Eng. 2012 Jul;40(7):1408-18. doi: 10.1007/s10439-012-0550-3. Epub 2012 Mar 27. Ann Biomed Eng. 2012. PMID: 22451256 Free PMC article.
-
Branched polyethylenimine-grafted-carboxymethyl chitosan copolymer enhances the delivery of pDNA or siRNA in vitro and in vivo.Int J Nanomedicine. 2013;8:3663-77. doi: 10.2147/IJN.S50911. Epub 2013 Sep 26. Int J Nanomedicine. 2013. PMID: 24106426 Free PMC article.
-
Poly(β-amino ester) nanoparticle delivery of TP53 has activity against small cell lung cancer in vitro and in vivo.Mol Cancer Ther. 2013 Apr;12(4):405-15. doi: 10.1158/1535-7163.MCT-12-0956. Epub 2013 Jan 30. Mol Cancer Ther. 2013. PMID: 23364678 Free PMC article.
-
Engineering DNA vaccines against infectious diseases.Acta Biomater. 2018 Oct 15;80:31-47. doi: 10.1016/j.actbio.2018.08.033. Epub 2018 Aug 31. Acta Biomater. 2018. PMID: 30172933 Free PMC article. Review.
References
Bibliography
-
- Pringle IA, Hyde SC, Gill DR. Non-viral vectors in cystic fibrosis gene therapy: recent developments and future prospects. Expert Opin. Biol. Ther. 2009;9(8):991–1003. - PubMed
-
- Sadelain M, Riviere I, Wang X, et al. Strategy for a multicenter Phase I clinical trial to evaluate globin gene transfer in β-thalassemia. Ann. NY Acad. Sci. 2010;1202:52–58. - PubMed
-
- Perumbeti A, Malik P. Therapy for β-globinopathies: a brief review and determinants for successful and safe correction. Ann. NY Acad. Sci. 2010;1202:36–44. - PubMed
-
- Viiala NO, Larsen SR, Rasko JE. Gene therapy for hemophilia: clinical trials and technical tribulations. Semin. Thromb. Hemost. 2009;35(1):81–92. - PubMed
Websites
-
- Gene Therapy Clinical Trials Worldwide. 2011 www.wiley.com/legacy/wileychi/genmed/clinical
-
- Schadendorf D. Gene therapy in patients with melanoma. Gene Therapy Clinical Trials Worldwide. www.abedia.com/wiley/record_detail. php?ID=149
-
- Mahvi D. Phase I/IB study of immunization with autologous tumor cells transfected with the GM-CSF gene by particle-mediated transfer in patients with melanoma or sarcoma. Gene Therapy Clinical Trials Worldwide. 1996 www.abedia.com/wiley/record_detail. php?ID=755 - PubMed
-
- Stewart DJ. Multicentre, randomized, double blind, placebo controlled trial of myocardial angiogenesis using VEGF165, intramyocardial gene delivery in patients with severe angina. Gene Therapy Clinical Trials Worldwide. 2002 www.abedia.com/wiley/record_detail. php?ID=68
-
- Ward M. The PHACeT Trial: pulmonary hypertension: assessment of cell therapy (Phase II) Gene Therapy Clinical Trials Worldwide. 2006 www.abedia.com/wiley/record_detail. php?ID=70
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
