N-terminal region of CusB is sufficient for metal binding and metal transfer with the metallochaperone CusF
- PMID: 22812620
- PMCID: PMC3448809
- DOI: 10.1021/bi300596a
N-terminal region of CusB is sufficient for metal binding and metal transfer with the metallochaperone CusF
Abstract
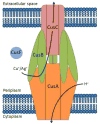
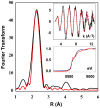
Gram-negative bacteria, such as Escherichia coli, utilize efflux resistance systems in order to expel toxins from their cells. Heavy-metal resistance is mediated by resistance nodulation cell division (RND)-based efflux pumps composed of a tripartite complex that includes an RND-transporter, an outer-membrane factor (OMF), and a membrane fusion protein (MFP) that spans the periplasmic space. MFPs are necessary for complex assembly and have been hypothesized to play an active role in substrate efflux. Crystal structures of MFPs are available, however incomplete, as large portions of the apparently disordered N- and C-termini are unresolved. Such is the case for CusB, the MFP of the E. coli Cu(I)/Ag(I) efflux pump CusCFBA. In this work, we have investigated the structure and function of the N-terminal region of CusB, which includes the metal-binding site and is missing from previously determined crystal structures. Results from mass spectrometry and X-ray absorption spectroscopy show that the isolated N-terminal 61 residues (CusB-NT) bind metal in a 1:1 stoichiometry with a coordination site composed of M21, M36, and M38, consistent with full-length CusB. NMR spectra show that CusB-NT is mostly disordered in the apo state; however, some slight structure is adopted upon metal binding. Much of the intact protein's function is maintained in this fragment as CusB-NT binds metal in vivo and in vitro, and metal is transferred between the metallochaperone CusF and CusB-NT in vitro. Functional analysis in vivo shows that full-length CusB is necessary in an intact polypeptide for full metal resistance, though CusB-NT alone can contribute partial metal resistance. These findings reinforce the theory that the role of CusB is not only to bind metal but also to play an active role in efflux.
Figures


Similar articles
-
Models for the Metal Transfer Complex of the N-Terminal Region of CusB and CusF.Biochemistry. 2015 Jul 14;54(27):4226-35. doi: 10.1021/acs.biochem.5b00195. Epub 2015 Jul 2. Biochemistry. 2015. PMID: 26079272
-
Interactions between CusF and CusB identified by NMR spectroscopy and chemical cross-linking coupled to mass spectrometry.Biochemistry. 2011 Apr 5;50(13):2559-66. doi: 10.1021/bi102012j. Epub 2011 Mar 8. Biochemistry. 2011. PMID: 21323389 Free PMC article.
-
EPR spectroscopy identifies Met and Lys residues that are essential for the interaction between the CusB N-terminal domain and metallochaperone CusF.Metallomics. 2015 Jul;7(7):1163-72. doi: 10.1039/c5mt00053j. Epub 2015 May 5. Metallomics. 2015. PMID: 25940871
-
The Cus efflux system removes toxic ions via a methionine shuttle.Protein Sci. 2011 Jan;20(1):6-18. doi: 10.1002/pro.532. Protein Sci. 2011. PMID: 20981744 Free PMC article. Review.
-
Metal export by CusCFBA, the periplasmic Cu(I)/Ag(I) transport system of Escherichia coli.Curr Top Membr. 2012;69:163-96. doi: 10.1016/B978-0-12-394390-3.00007-0. Curr Top Membr. 2012. PMID: 23046651 Review.
Cited by
-
NMR reveals the interplay between SilE and SilB model peptides in the context of silver resistance.Chem Commun (Camb). 2021 Sep 11;57(70):8726-8729. doi: 10.1039/d1cc02597j. Epub 2021 Aug 16. Chem Commun (Camb). 2021. PMID: 34396382 Free PMC article.
-
CopM is a novel copper-binding protein involved in copper resistance in Synechocystis sp. PCC 6803.Microbiologyopen. 2015 Feb;4(1):167-85. doi: 10.1002/mbo3.231. Epub 2014 Dec 26. Microbiologyopen. 2015. PMID: 25545960 Free PMC article.
-
Trapping intermediates in metal transfer reactions of the CusCBAF export pump of Escherichia coli.Commun Biol. 2018 Nov 14;1:192. doi: 10.1038/s42003-018-0181-9. eCollection 2018. Commun Biol. 2018. PMID: 30456313 Free PMC article.
-
Bacterial multidrug efflux transporters.Annu Rev Biophys. 2014;43:93-117. doi: 10.1146/annurev-biophys-051013-022855. Annu Rev Biophys. 2014. PMID: 24702006 Free PMC article. Review.
-
The battle for silver binding: How the interplay between the SilE, SilF, and SilB proteins contributes to the silver efflux pump mechanism.J Biol Chem. 2023 Aug;299(8):105004. doi: 10.1016/j.jbc.2023.105004. Epub 2023 Jul 1. J Biol Chem. 2023. PMID: 37394004 Free PMC article.
References
-
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. - PubMed
-
- Nies DH. Transition metal homeostasis in bacteria as a flow equilibrium of uptake and efflux processes. Amino Acids. 2009;37:22–22.
-
- Tikhonova EB, Zgurskaya HI. AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J Biol Chem. 2004;279:32116–32124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

