Dnt1 acts as a mitotic inhibitor of the spindle checkpoint protein dma1 in fission yeast
- PMID: 22809626
- PMCID: PMC3431938
- DOI: 10.1091/mbc.E11-12-1020
Dnt1 acts as a mitotic inhibitor of the spindle checkpoint protein dma1 in fission yeast
Abstract
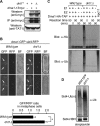
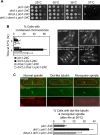
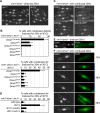
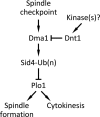
The Schizosaccharomyces pombe checkpoint protein Dma1 couples mitotic progression with cytokinesis and is important in delaying mitotic exit and cytokinesis when kinetochores are not properly attached to the mitotic spindle. Dma1 is a ubiquitin ligase and potential functional relative of the human tumor suppressor Chfr. Dma1 delays mitotic exit and cytokinesis by ubiquitinating a scaffold protein (Sid4) of the septation initiation network, which, in turn, antagonizes the ability of the Polo-like kinase Plo1 to promote cell division. Here we identify Dnt1 as a Dma1-binding protein. Several lines of evidence indicate that Dnt1 inhibits Dma1 function during metaphase. First, Dnt1 interacts preferentially with Dma1 during metaphase. Second, Dma1 ubiquitin ligase activity and Sid4 ubiquitination are elevated in dnt1 cells. Third, the enhanced mitotic defects in dnt1Δ plo1 double mutants are partially rescued by deletion of dma1(+), suggesting that the defects in dnt1 plo1 double mutants are attributable to excess Dma1 activity. Taken together, these data show that Dnt1 acts to restrain Dma1 activity in early mitosis to allow normal mitotic progression.
Figures


Similar articles
-
Relief of the Dma1-mediated checkpoint requires Dma1 autoubiquitination and dynamic localization.Mol Biol Cell. 2018 Sep 1;29(18):2176-2189. doi: 10.1091/mbc.E18-04-0261. Epub 2018 Jul 5. Mol Biol Cell. 2018. PMID: 29975113 Free PMC article.
-
Dma1 ubiquitinates the SIN scaffold, Sid4, to impede the mitotic localization of Plo1 kinase.EMBO J. 2011 Jan 19;30(2):341-54. doi: 10.1038/emboj.2010.317. Epub 2010 Dec 3. EMBO J. 2011. PMID: 21131906 Free PMC article.
-
CK1 is required for a mitotic checkpoint that delays cytokinesis.Curr Biol. 2013 Oct 7;23(19):1920-6. doi: 10.1016/j.cub.2013.07.077. Epub 2013 Sep 19. Curr Biol. 2013. PMID: 24055157 Free PMC article.
-
Spatiotemporal regulation of the Dma1-mediated mitotic checkpoint coordinates mitosis with cytokinesis.Curr Genet. 2019 Jun;65(3):663-668. doi: 10.1007/s00294-018-0921-x. Epub 2019 Jan 2. Curr Genet. 2019. PMID: 30600396 Free PMC article. Review.
-
Polar opposites: Fine-tuning cytokinesis through SIN asymmetry.Cytoskeleton (Hoboken). 2012 Oct;69(10):686-99. doi: 10.1002/cm.21044. Epub 2012 Jul 11. Cytoskeleton (Hoboken). 2012. PMID: 22786806 Free PMC article. Review.
Cited by
-
Relief of the Dma1-mediated checkpoint requires Dma1 autoubiquitination and dynamic localization.Mol Biol Cell. 2018 Sep 1;29(18):2176-2189. doi: 10.1091/mbc.E18-04-0261. Epub 2018 Jul 5. Mol Biol Cell. 2018. PMID: 29975113 Free PMC article.
-
Recovery from spindle checkpoint-mediated arrest requires a novel Dnt1-dependent APC/C activation mechanism.PLoS Genet. 2022 Sep 15;18(9):e1010397. doi: 10.1371/journal.pgen.1010397. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36108046 Free PMC article.
-
Heat stress-induced activation of MAPK pathway attenuates Atf1-dependent epigenetic inheritance of heterochromatin in fission yeast.Elife. 2024 Jan 30;13:e90525. doi: 10.7554/eLife.90525. Elife. 2024. PMID: 38289024 Free PMC article.
-
Fission yeast nucleolar protein Dnt1 regulates G2/M transition and cytokinesis by downregulating Wee1 kinase.J Cell Sci. 2013 Nov 1;126(Pt 21):4995-5004. doi: 10.1242/jcs.132845. Epub 2013 Sep 4. J Cell Sci. 2013. PMID: 24006256 Free PMC article.
-
Ubiquitination of CLIP-170 family protein restrains polarized growth upon DNA replication stress.Nat Commun. 2022 Sep 22;13(1):5565. doi: 10.1038/s41467-022-33311-y. Nat Commun. 2022. PMID: 36138017 Free PMC article.
References
-
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A 3rd, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998b;14:943–951. - PubMed
-
- Balasubramanian MK, McCollum D, Gould KL. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

