Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition
- PMID: 22804960
- PMCID: PMC3466155
- DOI: 10.1186/1471-2407-12-293
Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition
Abstract
Background: Clear cell renal cell carcinoma (ccRCC) accounts for more than 80% of the cases of renal cell carcinoma. In ccRCC deactivation of Von-Hippel-Lindau (VHL) gene contributes to the constitutive expression of hypoxia inducible factors 1 and 2 alpha (HIF-α), transcriptional regulators of several genes involved in tumor angiogenesis, glycolysis and drug resistance. We have demonstrated inhibition of HIF-1α by Se-Methylselenocysteine (MSC) via stabilization of prolyl hydroxylases 2 and 3 (PHDs) and a significant therapeutic synergy when combined with chemotherapy. This study was initiated to investigate the expression of PHDs, HIF-α, and VEGF-A in selected solid cancers, the mechanism of HIF-α inhibition by MSC, and to document antitumor activity of MSC against human ccRCC xenografts.
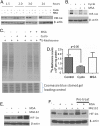
Methods: Tissue microarrays of primary human cancer specimens (ccRCC, head & neck and colon) were utilized to determine the incidence of PHD2/3, HIF-α, and VEGF-A by immunohistochemical methods. To investigate the mechanism(s) of HIF-α inhibition by MSC, VHL mutated ccRCC cells RC2 (HIF-1α positive), 786-0 (HIF-2α positive) and VHL wild type head & neck cancer cells FaDu (HIF-1α) were utilized. PHD2 and VHL gene specific siRNA knockdown and inhibitors of PHD2 and proteasome were used to determine their role in the degradation of HIF-1α by MSC.
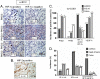
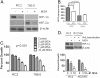
Results: We have demonstrated that ccRCC cells express low incidence of PHD2 (32%), undetectable PHD3, high incidence of HIF-α (92%), and low incidence of VEGF-A compared to head & neck and colon cancers. This laboratory was the first to identify MSC as a highly effective inhibitor of constitutively expressed HIF-α in ccRCC tumors. MSC did not inhibit HIF-1α protein synthesis, but facilitated its degradation. The use of gene knockdown and specific inhibitors confirmed that the inhibition of HIF-1α was PHD2 and proteasome dependent and VHL independent. The effects of MSC treatment on HIF-α were associated with significant antitumor activity against ccRCC xenograft.
Conclusions: Our results show the role of PHD2/3 in stable expression of HIF-α in human ccRCC. Furthermore, HIF-1α degradation by MSC is achieved through PHD2 dependent and VHL independent pathway which is unique for HIF-α regulation. These data provide the basis for combining MSC with currently used agents for ccRCC.
Figures


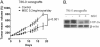
Similar articles
-
von Hippel-Lindau-dependent patterns of RNA polymerase II hydroxylation in human renal clear cell carcinomas.Clin Cancer Res. 2010 Nov 1;16(21):5142-52. doi: 10.1158/1078-0432.CCR-09-3416. Epub 2010 Oct 26. Clin Cancer Res. 2010. PMID: 20978146 Free PMC article.
-
Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases.Biochem J. 2004 Aug 1;381(Pt 3):761-7. doi: 10.1042/BJ20040620. Biochem J. 2004. PMID: 15104534 Free PMC article.
-
Prolyl Hydroxylase 3 Knockdown Accelerates VHL-Mutant Kidney Cancer Growth In Vivo.Int J Mol Sci. 2021 Mar 11;22(6):2849. doi: 10.3390/ijms22062849. Int J Mol Sci. 2021. PMID: 33799686 Free PMC article.
-
Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer.Eur Urol. 2016 Apr;69(4):646-657. doi: 10.1016/j.eururo.2015.08.007. Epub 2015 Aug 19. Eur Urol. 2016. PMID: 26298207 Free PMC article. Review.
-
A Precision Strategy to Cure Renal Cell Carcinoma by Targeting Transglutaminase 2.Int J Mol Sci. 2020 Apr 3;21(7):2493. doi: 10.3390/ijms21072493. Int J Mol Sci. 2020. PMID: 32260198 Free PMC article. Review.
Cited by
-
Non-Coding Micro RNAs and Hypoxia-Inducible Factors Are Selenium Targets for Development of a Mechanism-Based Combination Strategy in Clear-Cell Renal Cell Carcinoma-Bench-to-Bedside Therapy.Int J Mol Sci. 2018 Oct 29;19(11):3378. doi: 10.3390/ijms19113378. Int J Mol Sci. 2018. PMID: 30380599 Free PMC article.
-
Methylseleninic acid elevates REDD1 and inhibits prostate cancer cell growth despite AKT activation and mTOR dysregulation in hypoxia.Cancer Med. 2014 Apr;3(2):252-64. doi: 10.1002/cam4.198. Epub 2014 Feb 7. Cancer Med. 2014. PMID: 24515947 Free PMC article.
-
Chemical activation of prolyl hydroxylase-2 by BBAP-1 down regulates hypoxia inducible factor-1α and fatty acid synthase for mammary gland chemoprevention.RSC Adv. 2018 Apr 4;8(23):12848-12860. doi: 10.1039/c8ra01239c. eCollection 2018 Apr 3. RSC Adv. 2018. PMID: 35541235 Free PMC article.
-
Targeting EGFR in Combination with Nutritional Supplements on Antitumor Efficacy in a Lung Cancer Mouse Model.Mar Drugs. 2022 Nov 29;20(12):751. doi: 10.3390/md20120751. Mar Drugs. 2022. PMID: 36547898 Free PMC article.
-
Constitutive expression of HIF-α plays a major role in generation of clear-cell phenotype in human primary and metastatic renal carcinoma.Appl Immunohistochem Mol Morphol. 2014 Oct;22(9):642-7. doi: 10.1097/PAI.0000000000000012. Appl Immunohistochem Mol Morphol. 2014. PMID: 25046225 Free PMC article.
References
-
- US National Institutes of Health. Institute N.C. kidney cancer [online] 2010. http://www.cancer.gov/cancertopics/types/kidney.
-
- Hervouet E, Demont J, Pecina P, Vojtiskova A, Houstek J, Simonnet H, Godinot C. A new role for the von Hippel-Lindau tumor suppressor protein: stimulation of mitochondrial oxidative phosphorylation complex biogenesis. Carcinogenesis. 2005;26(3):531–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

