Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia
- PMID: 22789851
- PMCID: PMC3408820
- DOI: 10.1016/j.bbrc.2012.07.008
Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia
Abstract
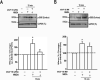
A-Kinase anchoring protein 150 (AKAP150) is required for the phosphorylation of transient receptor potential cation channel subfamily V member 1 (TRPV1) by PKA or PKC in sensory neurons and, hence, affects TRPV1-dependent hyperalgesia under pathological conditions. Recently, we showed that the activation of N-methyl-D-aspartate (NMDA) receptors sensitizes TRPV1 by enhancing serine phosphorylation through PKC in trigeminal nociceptors. In this study, we extended this observation by investigating whether AKAP150 mediates NMDA-induced phosphorylation of TRPV1 via PKC in native sensory neurons in the rat. By adopting a phospho-specific antibody combined with a surface biotinylation assay, we first assessed NMDA-induced changes in the phosphorylation level of serine 800 residues (S800) in TRPV1 delimited to cell surface membrane in cultured trigeminal ganglia (TG). The biotinylation assay yielded that the application of NMDA significantly increased the phosphorylation of S800 (p-S800) of TRPV1 at time points correlating with the development of NMDA-induced mechanical hyperalgesia [10]. We then obtained a siRNA sequence against AKAP150 that dose-dependently down-regulated the AKAP150 protein. Pretreatment of TG culture with the siRNA, but not mismatch sequences, prevented the NMDA-induced phosphorylation of serine residues of total TRPV1 as well as S800 of membrane bound TRPV1. We confirmed that AKAP150 co-immunoprecipitated with TRPV1 and demonstrated that it also co-immunoprecipitated with NMDA receptor subunits (NR1 and NR2B) in TG. These data offer novel information that the activation of NMDA-induced TRPV1 sensitization involves p-S800 of TRPV1 in cell surface membrane in native sensory neurons and that AKAP150 is required for NMDA-and PKC-mediated phosphorylation of TRPV1 S800. Therefore, we propose that the NMDA receptor, AKAP150, and TRPV1 forms a signaling complex that underlies the sensitization of trigeminal nociceptors by modulating phosphorylation of specific TRPV1 residues.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
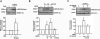
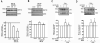
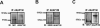
Similar articles
-
Peripheral group I metabotropic glutamate receptor activation leads to muscle mechanical hyperalgesia through TRPV1 phosphorylation in the rat.J Pain. 2015 Jan;16(1):67-76. doi: 10.1016/j.jpain.2014.10.008. Epub 2014 Nov 1. J Pain. 2015. PMID: 25451626 Free PMC article.
-
A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1.Pain. 2009 Dec;146(3):301-307. doi: 10.1016/j.pain.2009.08.002. Epub 2009 Sep 19. Pain. 2009. PMID: 19767149 Free PMC article.
-
A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1.Pain. 2008 Sep 15;138(3):604-616. doi: 10.1016/j.pain.2008.02.022. Epub 2008 Apr 1. Pain. 2008. PMID: 18381233 Free PMC article.
-
Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle.Pain. 2012 Jul;153(7):1514-1524. doi: 10.1016/j.pain.2012.04.015. Epub 2012 May 19. Pain. 2012. PMID: 22609428 Free PMC article.
-
PP2B/calcineurin-mediated desensitization of TRPV1 does not require AKAP150.Biochem J. 2010 Dec 15;432(3):549-56. doi: 10.1042/BJ20100936. Biochem J. 2010. PMID: 20883208 Free PMC article.
Cited by
-
Electroacupuncture inhibits the interaction between peripheral TRPV1 and P2X3 in rats with different pathological pain.Physiol Res. 2021 Aug 31;70(4):635-647. doi: 10.33549/physiolres.934649. Epub 2021 Jun 1. Physiol Res. 2021. PMID: 34062076 Free PMC article.
-
Involvement of mGluR5 and TRPV1 in visceral nociception in a rat model of uterine cervical distension.Mol Pain. 2018 Jan-Dec;14:1744806918816850. doi: 10.1177/1744806918816850. Epub 2018 Nov 16. Mol Pain. 2018. PMID: 30444177 Free PMC article.
-
Studies on a novel regimen for management of orofacial pain and morphine tolerance.J Dent Sci. 2018 Jun;13(2):131-137. doi: 10.1016/j.jds.2017.08.006. Epub 2017 Nov 10. J Dent Sci. 2018. PMID: 30895108 Free PMC article.
-
Peripheral group I metabotropic glutamate receptor activation leads to muscle mechanical hyperalgesia through TRPV1 phosphorylation in the rat.J Pain. 2015 Jan;16(1):67-76. doi: 10.1016/j.jpain.2014.10.008. Epub 2014 Nov 1. J Pain. 2015. PMID: 25451626 Free PMC article.
-
H89 dihydrochloride hydrate and calphostin C lower the body temperature through TRPV1.Mol Med Rep. 2018 Jan;17(1):1599-1608. doi: 10.3892/mmr.2017.8078. Epub 2017 Nov 15. Mol Med Rep. 2018. PMID: 29257197 Free PMC article.
References
-
- Caterina MJ, Schmacher, Tominaga M, Rosen TA, Levine JD. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. - PubMed
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. - PubMed
-
- Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011;704:615–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

