Cell-free transmission of human adenovirus by passive mass transfer in cell culture simulated in a computer model
- PMID: 22787215
- PMCID: PMC3446567
- DOI: 10.1128/JVI.01102-12
Cell-free transmission of human adenovirus by passive mass transfer in cell culture simulated in a computer model
Abstract
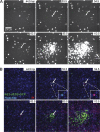
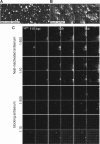
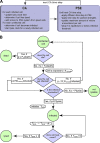
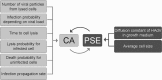
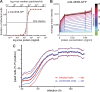
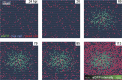
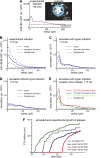
Viruses spread between cells, tissues, and organisms by cell-free and cell-cell transmissions. Both mechanisms enhance disease development, but it is difficult to distinguish between them. Here, we analyzed the transmission mode of human adenovirus (HAdV) in monolayers of epithelial cells by wet laboratory experimentation and a computer simulation. Using live-cell fluorescence microscopy and replication-competent HAdV2 expressing green fluorescent protein, we found that the spread of infection invariably occurred after cell lysis. It was affected by convection and blocked by neutralizing antibodies but was independent of second-round infections. If cells were overlaid with agarose, convection was blocked and round plaques developed around lytic infected cells. Infected cells that did not lyse did not give rise to plaques, highlighting the importance of cell-free transmission. Key parameters for cell-free virus transmission were the time from infection to lysis, the dose of free viruses determining infection probability, and the diffusion of single HAdV particles in aqueous medium. With these parameters, we developed an in silico model using multiscale hybrid dynamics, cellular automata, and particle strength exchange. This so-called white box model is based on experimentally determined parameters and reproduces viral infection spreading as a function of the local concentration of free viruses. These analyses imply that the extent of lytic infections can be determined by either direct plaque assays or can be predicted by calculations of virus diffusion constants and modeling.
Figures

Similar articles
-
Expanding the adenovirus toolbox: reporter viruses for studying the dynamics of human adenovirus replication.J Virol. 2024 May 14;98(5):e0020724. doi: 10.1128/jvi.00207-24. Epub 2024 Apr 19. J Virol. 2024. PMID: 38639487 Free PMC article.
-
The FDA-Approved Drug Nelfinavir Inhibits Lytic Cell-Free but Not Cell-Associated Nonlytic Transmission of Human Adenovirus.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01002-20. doi: 10.1128/AAC.01002-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32601166 Free PMC article.
-
Development of plaque assays for adenoviruses 40 and 41.J Virol Methods. 2008 Jul;151(1):140-5. doi: 10.1016/j.jviromet.2008.03.007. Epub 2008 Apr 25. J Virol Methods. 2008. PMID: 18440077
-
Viral and cellular interactions during adenovirus DNA replication.FEBS Lett. 2019 Dec;593(24):3531-3550. doi: 10.1002/1873-3468.13695. Epub 2019 Dec 17. FEBS Lett. 2019. PMID: 31764999 Free PMC article. Review.
-
Adenoviral inhibitors of apoptotic cell death.Virus Res. 2002 Sep;88(1-2):87-101. doi: 10.1016/s0168-1702(02)00122-3. Virus Res. 2002. PMID: 12297329 Review.
Cited by
-
Human Adenovirus Type 5 Infection Leads to Nuclear Envelope Destabilization and Membrane Permeability Independently of Adenovirus Death Protein.Int J Mol Sci. 2021 Dec 2;22(23):13034. doi: 10.3390/ijms222313034. Int J Mol Sci. 2021. PMID: 34884837 Free PMC article.
-
Cell-to-cell transmission of viruses.Curr Opin Virol. 2013 Feb;3(1):44-50. doi: 10.1016/j.coviro.2012.11.004. Epub 2012 Dec 5. Curr Opin Virol. 2013. PMID: 23219376 Free PMC article. Review.
-
The UPR sensor IRE1α and the adenovirus E3-19K glycoprotein sustain persistent and lytic infections.Nat Commun. 2020 Apr 24;11(1):1997. doi: 10.1038/s41467-020-15844-2. Nat Commun. 2020. PMID: 32332742 Free PMC article.
-
Concepts in Light Microscopy of Viruses.Viruses. 2018 Apr 18;10(4):202. doi: 10.3390/v10040202. Viruses. 2018. PMID: 29670029 Free PMC article. Review.
-
Preexisting cell state rather than stochastic noise confers high or low infection susceptibility of human lung epithelial cells to adenovirus.mSphere. 2024 Oct 29;9(10):e0045424. doi: 10.1128/msphere.00454-24. Epub 2024 Sep 24. mSphere. 2024. PMID: 39315811 Free PMC article.
References
-
- Bankhead A, et al. 2011. A simulation framework to investigate in vitro viral infection dynamics. J. Comput. Sci. doi:10.1016/j.jocs.2011.08.007 - DOI - PMC - PubMed
-
- Barton KN, et al. 2006. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol. Ther. 13:347–356 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

