Heparan sulfate is an attachment factor for foamy virus entry
- PMID: 22787203
- PMCID: PMC3446549
- DOI: 10.1128/JVI.00051-12
Heparan sulfate is an attachment factor for foamy virus entry
Abstract
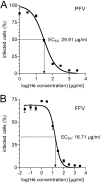
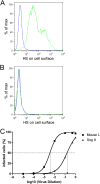
The cellular receptor of foamy viruses (FVs) is unknown. The broad spectrum of permissive cells suggests that the cellular receptor is a molecular structure with almost ubiquitous prevalence. Here, we investigated the ability of heparan sulfate (HS), a glycosaminoglycan (GAG) present on the extracellular matrix of many cells, to bind FV particles and to permit prototype FV (PFV) and feline FV (FFV) entry. Permissivity of different cell lines for FV entry correlated with the amount of heparan sulfate present on the cell surface. The resulting 50% cell culture infectious doses (CCID(50)s) were distributed over a range of 4 logs, which means that the most susceptible cell line tested (HT1080) was more than 10,000 times more susceptible for PFV infection than the least susceptible cell line (CRL-2242). HS surface expression varied over a range of 2 logs. HS expression and FV susceptibility were positively correlated (P < 0.001). Enzymatic digestion of heparan sulfate on HT1080 cells diminished permissivity for PFV entry by a factor of at least 500. Using fast protein liquid chromatography (FPLC), we demonstrated binding of FV vector particles to a gel filtration column packed with heparin, a molecule structurally related to heparan sulfate, allowing for the purification of infectious particles. Both PFV and FFV infection were inhibited by soluble heparin. Our results show that FVs bind to HS and that this interaction is a pivotal step for viral entry, suggesting that HS is a cellular attachment factor for FVs.
Figures

Similar articles
-
Cell Membrane-associated heparan sulfate is a receptor for prototype foamy virus in human, monkey, and rodent cells.Mol Ther. 2012 Jun;20(6):1158-66. doi: 10.1038/mt.2012.41. Epub 2012 Mar 20. Mol Ther. 2012. PMID: 22434139 Free PMC article.
-
Differential pH-dependent cellular uptake pathways among foamy viruses elucidated using dual-colored fluorescent particles.Retrovirology. 2012 Aug 30;9:71. doi: 10.1186/1742-4690-9-71. Retrovirology. 2012. PMID: 22935135 Free PMC article.
-
Feline foamy virus genome and replication strategy.J Virol. 2003 Nov;77(21):11324-31. doi: 10.1128/jvi.77.21.11324-11331.2003. J Virol. 2003. PMID: 14557618 Free PMC article.
-
Early events in foamy virus-host interaction and intracellular trafficking.Viruses. 2013 Apr 8;5(4):1055-74. doi: 10.3390/v5041055. Viruses. 2013. PMID: 23567621 Free PMC article. Review.
-
Heparan sulfate: anchor for viral intruders?Biochimie. 2001 Aug;83(8):811-7. doi: 10.1016/s0300-9084(01)01290-1. Biochimie. 2001. PMID: 11530214 Review.
Cited by
-
Foamy Viruses, Bet, and APOBEC3 Restriction.Viruses. 2021 Mar 18;13(3):504. doi: 10.3390/v13030504. Viruses. 2021. PMID: 33803830 Free PMC article. Review.
-
Emerging Roles of Heparanase in Viral Pathogenesis.Pathogens. 2017 Sep 18;6(3):43. doi: 10.3390/pathogens6030043. Pathogens. 2017. PMID: 28927006 Free PMC article. Review.
-
FV Vectors as Alternative Gene Vehicles for Gene Transfer in HSCs.Viruses. 2020 Mar 19;12(3):332. doi: 10.3390/v12030332. Viruses. 2020. PMID: 32204324 Free PMC article. Review.
-
Discovery of prosimian and afrotherian foamy viruses and potential cross species transmissions amidst stable and ancient mammalian co-evolution.Retrovirology. 2014 Aug 4;11:61. doi: 10.1186/1742-4690-11-61. Retrovirology. 2014. PMID: 25091111 Free PMC article.
-
Structures of the Foamy virus fusion protein reveal an unexpected link with the F protein of paramyxo- and pneumoviruses.Sci Adv. 2024 Oct 11;10(41):eado7035. doi: 10.1126/sciadv.ado7035. Epub 2024 Oct 11. Sci Adv. 2024. PMID: 39392890 Free PMC article.
References
-
- Achong BG, Mansell PW, Epstein MA, Clifford P. 1971. An unusual virus in cultures from a human nasopharyngeal carcinoma. J. Natl. Cancer Inst. 46:299–307 - PubMed
-
- Bernfield M, et al. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729–777 - PubMed
-
- Betsem E, Rua R, Tortevoye P, Froment A, Gessain A. 2011. Frequent and recent human acquisition of simian foamy viruses through apes' bites in central Africa. PLoS Pathog. 7:e1002306 doi:10.1371/journal.ppat.1002306 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

