The Werner syndrome protein promotes CAG/CTG repeat stability by resolving large (CAG)(n)/(CTG)(n) hairpins
- PMID: 22787159
- PMCID: PMC3436269
- DOI: 10.1074/jbc.M112.389791
The Werner syndrome protein promotes CAG/CTG repeat stability by resolving large (CAG)(n)/(CTG)(n) hairpins
Abstract
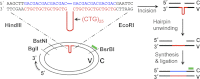
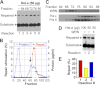
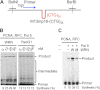
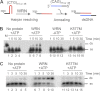
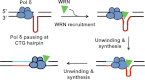
Expansion of CAG/CTG repeats causes certain neurological and neurodegenerative disorders, and the formation and subsequent persistence of stable DNA hairpins within these repeats are believed to contribute to CAG/CTG repeat instability. Human cells possess a DNA hairpin repair (HPR) pathway, which removes various (CAG)(n) and (CTG)(n) hairpins in a nick-directed and strand-specific manner. Interestingly, this HPR system processes a (CTG)(n) hairpin on the template DNA strand much less efficiently than a (CAG)(n) hairpin on the same strand (Hou, C., Chan, N. L., Gu, L., and Li, G. M. (2009) Incision-dependent and error-free repair of (CAG)(n)/(CTG)(n) hairpins in human cell extracts. Nat. Struct. Mol. Biol. 16, 869-875), suggesting the involvement of an additional component for (CTG)(n) HPR. To identify this activity, a functional in vitro HPR assay was used to screen partially purified HeLa nuclear fractions for their ability to stimulate (CTG)(n) HPR. We demonstrate here that the stimulating activity is the Werner syndrome protein (WRN). Although WRN contains both a 3'→5' helicase activity and a 3'→5' exonuclease activity, the stimulating activity was found to be the helicase activity, as a WRN helicase mutant failed to enhance (CTG)(n) HPR. Consistently, WRN efficiently unwound large (CTG)(n) hairpins and promoted DNA polymerase δ-catalyzed DNA synthesis using a (CTG)(n) hairpin as a template. We, therefore, conclude that WRN stimulates (CTG)(n) HPR on the template DNA strand by resolving the hairpin so that it can be efficiently used as a template for repair or replicative synthesis.
Figures
Similar articles
-
Coordinated processing of 3' slipped (CAG)n/(CTG)n hairpins by DNA polymerases β and δ preferentially induces repeat expansions.J Biol Chem. 2013 May 24;288(21):15015-22. doi: 10.1074/jbc.M113.464370. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585564 Free PMC article.
-
MutSβ promotes trinucleotide repeat expansion by recruiting DNA polymerase β to nascent (CAG)n or (CTG)n hairpins for error-prone DNA synthesis.Cell Res. 2016 Jul;26(7):775-86. doi: 10.1038/cr.2016.66. Epub 2016 Jun 3. Cell Res. 2016. PMID: 27255792 Free PMC article.
-
In vitro repair of DNA hairpins containing various numbers of CAG/CTG trinucleotide repeats.DNA Repair (Amst). 2012 Feb 1;11(2):201-9. doi: 10.1016/j.dnarep.2011.10.020. Epub 2011 Oct 29. DNA Repair (Amst). 2012. PMID: 22041023 Free PMC article.
-
The WRN helicase: resolving a new target in microsatellite unstable cancers.Curr Opin Genet Dev. 2021 Dec;71:34-38. doi: 10.1016/j.gde.2021.06.014. Epub 2021 Jul 17. Curr Opin Genet Dev. 2021. PMID: 34284257 Review.
-
Structural and Dynamical Properties of Nucleic Acid Hairpins Implicated in Trinucleotide Repeat Expansion Diseases.Biomolecules. 2024 Oct 10;14(10):1278. doi: 10.3390/biom14101278. Biomolecules. 2024. PMID: 39456210 Free PMC article. Review.
Cited by
-
Repeat instability during DNA repair: Insights from model systems.Crit Rev Biochem Mol Biol. 2015 Mar-Apr;50(2):142-67. doi: 10.3109/10409238.2014.999192. Epub 2015 Jan 22. Crit Rev Biochem Mol Biol. 2015. PMID: 25608779 Free PMC article. Review.
-
FAN1, a DNA Repair Nuclease, as a Modifier of Repeat Expansion Disorders.J Huntingtons Dis. 2021;10(1):95-122. doi: 10.3233/JHD-200448. J Huntingtons Dis. 2021. PMID: 33579867 Free PMC article. Review.
-
Coordinated processing of 3' slipped (CAG)n/(CTG)n hairpins by DNA polymerases β and δ preferentially induces repeat expansions.J Biol Chem. 2013 May 24;288(21):15015-22. doi: 10.1074/jbc.M113.464370. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585564 Free PMC article.
-
Mechanisms of genetic instability caused by (CGG)n repeats in an experimental mammalian system.Nat Struct Mol Biol. 2018 Aug;25(8):669-676. doi: 10.1038/s41594-018-0094-9. Epub 2018 Jul 30. Nat Struct Mol Biol. 2018. PMID: 30061600 Free PMC article.
-
DNA helicases involved in DNA repair and their roles in cancer.Nat Rev Cancer. 2013 Aug;13(8):542-58. doi: 10.1038/nrc3560. Epub 2013 Jul 11. Nat Rev Cancer. 2013. PMID: 23842644 Free PMC article. Review.
References
-
- López Castel A., Cleary J. D., Pearson C. E. (2010) Repeat instability as the basis for human diseases and as a potential target for therapy. Nat. Rev. Mol. Cell Biol. 11, 165–170 - PubMed
-
- Pearson C. E., Nichol Edamura K., Cleary J. D. (2005) Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 6, 729–742 - PubMed
-
- Mirkin S. M. (2007) Expandable DNA repeats and human disease. Nature 447, 932–940 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

