Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity
- PMID: 22783257
- PMCID: PMC3388714
- DOI: 10.3389/fimmu.2012.00183
Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity
Abstract
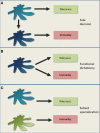
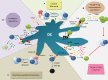
The last decades of Nobel prize-honored research have unequivocally proven a key role of dendritic cells (DCs) at controlling both T cell immunity and tolerance. A tight balance between these opposing DC functions ensures immune homeostasis and host integrity. Its perturbation could explain pathological conditions such as the attack of self tissues, chronic infections, and tumor immune evasion. While recent insights into the complex DC network help to understand the contribution of individual DC subsets to immunity, the tolerogenic functions of DCs only begin to emerge. As these consist of many different layers, the definition of a "tolerogenic DC" is subjected to variation. Moreover, the implication of DCs and DC subsets in the suppression of autoimmunity are incompletely resolved. In this review, we point out conceptual controversies and dissect the various layers of DC-mediated T cell tolerance. These layers include central tolerance, Foxp3(+) regulatory T cells (Tregs), anergy/deletion and negative feedback regulation. The mode and kinetics of antigen presentation is highlighted as an additional factor shaping tolerance. Special emphasis is given to the interaction between layers of tolerance as well as their differential regulation during inflammation. Furthermore, potential technical caveats of DC depletion models are considered. Finally, we summarize our current understanding of DC-mediated tolerance and its role for the suppression of autoimmunity. Understanding the mechanisms of DC-mediated tolerance and their complex interplay is fundamental for the development of selective therapeutic strategies, e.g., for the modulation of autoimmune responses or for the immunotherapy of cancer.
Keywords: CD103; DC; Foxp3; Treg; autoimmunity; infection; tolerance.
Figures

Similar articles
-
Functional crosstalk between dendritic cells and Foxp3(+) regulatory T cells in the maintenance of immune tolerance.Front Immunol. 2012 Jun 22;3:165. doi: 10.3389/fimmu.2012.00165. eCollection 2012. Front Immunol. 2012. PMID: 22737152 Free PMC article.
-
Regulatory dendritic cells in autoimmunity: A comprehensive review.J Autoimmun. 2015 Sep;63:1-12. doi: 10.1016/j.jaut.2015.07.011. Epub 2015 Aug 5. J Autoimmun. 2015. PMID: 26255250 Review.
-
The benefits of diversity: heterogenous DC populations allow for both immunity and tolerance.J Theor Biol. 2014 Sep 21;357:86-102. doi: 10.1016/j.jtbi.2014.04.034. Epub 2014 May 9. J Theor Biol. 2014. PMID: 24816181
-
Role of Dendritic Cells in the Induction of Lymphocyte Tolerance.Front Immunol. 2015 Oct 20;6:535. doi: 10.3389/fimmu.2015.00535. eCollection 2015. Front Immunol. 2015. PMID: 26539197 Free PMC article. Review.
-
Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo.J Immunol. 2008 Sep 1;181(5):3137-47. doi: 10.4049/jimmunol.181.5.3137. J Immunol. 2008. Retraction in: J Immunol. 2010 Jun 1;184(11):6556. doi: 10.4049/jimmunol.1090035 PMID: 18713984 Free PMC article. Retracted.
Cited by
-
Immunostimulatory Effects Triggered by Enterococcus faecalis CECT7121 Probiotic Strain Involve Activation of Dendritic Cells and Interferon-Gamma Production.PLoS One. 2015 May 15;10(5):e0127262. doi: 10.1371/journal.pone.0127262. eCollection 2015. PLoS One. 2015. PMID: 25978357 Free PMC article.
-
Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease.Annu Rev Immunol. 2014;32:83-119. doi: 10.1146/annurev-immunol-032713-120249. Epub 2013 Dec 18. Annu Rev Immunol. 2014. PMID: 24364806 Free PMC article. Review.
-
Tolerizing Strategies for the Treatment of Autoimmune Diseases: From ex vivo to in vivo Strategies.Front Immunol. 2020 May 14;11:674. doi: 10.3389/fimmu.2020.00674. eCollection 2020. Front Immunol. 2020. PMID: 32477325 Free PMC article. Review.
-
Regulation of lung immunity by dendritic cells: Implications for asthma, chronic obstructive pulmonary disease and infectious disease.Innate Immun. 2019 Aug;25(6):326-336. doi: 10.1177/1753425918821732. Innate Immun. 2019. PMID: 31291810 Free PMC article. Review.
-
Self-antigen presentation by dendritic cells in autoimmunity.Front Immunol. 2014 Feb 13;5:55. doi: 10.3389/fimmu.2014.00055. eCollection 2014. Front Immunol. 2014. PMID: 24592266 Free PMC article. Review.
References
-
- Anz D., Koelzer V. H., Moder S., Thaler R., Schwerd T., Lahl K., Sparwasser T., Besch R., Poeck H., Hornung V., Hartmann G., Rothenfusser S., Bourquin C., Endres S. (2010). Immunostimulatory RNA blocks suppression by regulatory T cells. J. Immunol. 184, 939–946 10.4049/jimmunol.0901245 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

