AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies
- PMID: 22771770
- PMCID: PMC3408547
- DOI: 10.1016/j.neuropharm.2012.06.046
AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies
Abstract
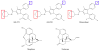
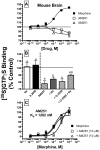
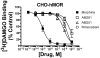
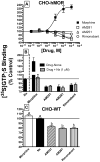
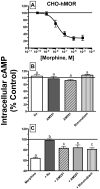
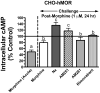
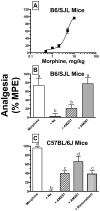
Mu-opioid and CB1-cannabinoid agonists produce analgesia; however, adverse effects limit use of drugs in both classes. Additive or synergistic effects resulting from concurrent administration of low doses of mu- and CB1-agonists may produce analgesia with fewer side effects. Synergism potentially results from interaction between mu-opioid receptors (MORs) and CB1 receptors (CB1Rs). AM-251 and rimonabant are CB1R antagonist/inverse agonists employed to validate opioid-cannabinoid interactions, presumed to act selectively at CB1Rs. Therefore, the potential for direct action of these antagonists at MORs is rarely considered. This study determined if AM-251 and/or rimonabant directly bind and modulate the function of MORs. Surprisingly, AM-251 and rimonabant, but not a third CB1R inverse agonist AM-281, bind with mid-nanomolar affinity to human MORs with a rank order of affinity (K(i)) of AM-251 (251 nM) > rimonabant (652 nM) > AM281 (2135 nM). AM-251 and rimonabant, but not AM-281, also competitively antagonize morphine induced G-protein activation in CHO-hMOR cell homogenates (K(b) = 719 or 1310 nM, respectively). AM-251 and rimonabant block morphine inhibition of cAMP production, while only AM-251 elicits cAMP rebound in CHO-hMOR cells chronically exposed to morphine. AM-251 and rimonabant (10 mg/kg) attenuate morphine analgesia, whereas the same dose of AM-281 produces little effect. Therefore, in addition to high CB1R affinity, AM-251 and rimonabant bind to MORs with mid-nanomolar affinity and at higher doses may affect morphine analgesia via direct antagonism at MORs. Such CB1-independent of these antagonists effects may contribute to reported inconsistencies when CB1/MOR interactions are examined via pharmacological methods in CB1-knockout versus wild-type mice.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Low dosage of rimonabant leads to anxiolytic-like behavior via inhibiting expression levels and G-protein activity of kappa opioid receptors in a cannabinoid receptor independent manner.Neuropharmacology. 2015 Feb;89:298-307. doi: 10.1016/j.neuropharm.2014.10.008. Neuropharmacology. 2015. PMID: 25446673
-
Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro.Br J Pharmacol. 2007 Mar;150(5):613-23. doi: 10.1038/sj.bjp.0707133. Epub 2007 Jan 22. Br J Pharmacol. 2007. PMID: 17245363 Free PMC article.
-
Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor.Mol Pharmacol. 2006 Jul;70(1):41-50. doi: 10.1124/mol.105.020552. Epub 2006 Mar 29. Mol Pharmacol. 2006. PMID: 16571654
-
CB₁-independent mechanisms of Δ⁹-THCV, AM251 and SR141716 (rimonabant).J Clin Pharm Ther. 2012 Jun;37(3):260-5. doi: 10.1111/j.1365-2710.2011.01284.x. Epub 2011 Jul 11. J Clin Pharm Ther. 2012. PMID: 21740450 Review.
-
Pharmacophores for ligand recognition and activation/inactivation of the cannabinoid receptors.Curr Pharm Des. 2003;9(20):1607-33. doi: 10.2174/1381612033454577. Curr Pharm Des. 2003. PMID: 12871061 Review.
Cited by
-
Anxiety Modulation by Cannabinoids-The Role of Stress Responses and Coping.Int J Mol Sci. 2023 Oct 30;24(21):15777. doi: 10.3390/ijms242115777. Int J Mol Sci. 2023. PMID: 37958761 Free PMC article. Review.
-
CB1 and CB2 receptors are novel molecular targets for Tamoxifen and 4OH-Tamoxifen.Biochem Biophys Res Commun. 2013 Nov 15;441(2):339-43. doi: 10.1016/j.bbrc.2013.10.057. Epub 2013 Oct 19. Biochem Biophys Res Commun. 2013. PMID: 24148245 Free PMC article.
-
Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents.Addict Biol. 2020 Jul;25(4):e12783. doi: 10.1111/adb.12783. Epub 2019 Jun 19. Addict Biol. 2020. PMID: 31215752 Free PMC article.
-
The Neuroprotective Effect of Lithium in cannabinoid Dependence is Mediated through Modulation of Cyclic AMP, ERK1/2 and GSK-3β Phosphorylation in Cerebellar Granular Neurons of Rat.Iran J Pharm Res. 2015 Fall;14(4):1123-35. Iran J Pharm Res. 2015. PMID: 26664379 Free PMC article.
-
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia.bioRxiv [Preprint]. 2024 Apr 2:2024.04.02.585967. doi: 10.1101/2024.04.02.585967. bioRxiv. 2024. Update in: Sci Adv. 2024 Nov 29;10(48):eadq4779. doi: 10.1126/sciadv.adq4779 PMID: 38766079 Free PMC article. Updated. Preprint.
References
-
- Avidor-Reiss T, Bayewitch M, Levy R, Matus-Leibovitch N, Nevo I, Vogel Z. Adenylyl cyclase supersensitization in mu-opioid receptor-transfected Chinese hamster ovary cells following chronic opioid treatment. J Biol Chem. 1995;270:29732–29738. - PubMed
-
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

