Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development
- PMID: 22771579
- PMCID: PMC3423513
- DOI: 10.1016/j.ydbio.2012.06.023
Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development
Abstract
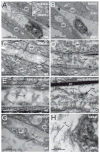
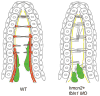
Hemicentin 1 (Hmcn1) and Hemicentin 2 (Hmcn2) belong to the fibulin family of extracellular matrix (ECM) proteins that play pivotal roles during development and homeostasis of a variety of vertebrate tissues. Recently, we have shown that mutations in zebrafish Hmcn1, also called Fibulin 6, lead to massive fin blistering, similar to the defects caused by the Fraser syndrome gene Fras1. In contrast, the role of Hmcn2 during vertebrate development has thus far been uncharacterized. In zebrafish, hmcn2, like fibulin 1 (fbln1), another member of the fibulin family, is predominantly expressed in fin mesenchymal cells and developing somites, contrasting the strict epithelial expression of hmcn1. While antisense morpholino oligonucleotide (MO)-based knockdown of hmcn2 did not yield any discernable defects, hmcn2/fbln1 double knockdown fish displayed blistering in the trunk, pointing to an essential contribution of these proteins from mesodermal sources for proper epidermal-dermal junction formation. In contrast, and unlike hmcn1 mutants, epidermal-dermal junctions in the fin folds of hmcn2/fbln1 double knockdown fish were only moderately affected. Instead, they displayed impaired migration of fin mesenchymal cells into the fin folds, pointing to a crucial role of Hmcn2 and Fbln1 to remodel the ECM of the fin fold interepidermal space, which is a prerequisite for fibroblast ingrowth. TEM analyses suggest that this ECM remodeling occurs at the level of actinotrichia, the collageneous migration substrate of mesenchymal cells, and at the level of cross fibers, which resemble mammalian microfibers. This work provides first insights into the role of Hmcn2 during vertebrate development, identifying it as an evolutionary conserved protein that acts in functional redundancy with Fbln1C and/or Fbln1D isoforms to regulate tissue adhesion and cell migration, while extending the current knowledge of the functions of vertebrate Fbln1.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Hemicentin-1 is an essential extracellular matrix component of the dermal-epidermal and myotendinous junctions.Sci Rep. 2021 Sep 9;11(1):17926. doi: 10.1038/s41598-021-96824-4. Sci Rep. 2021. PMID: 34504132 Free PMC article.
-
Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 as potential novel fraser syndrome disease genes.PLoS Genet. 2010 Apr 15;6(4):e1000907. doi: 10.1371/journal.pgen.1000907. PLoS Genet. 2010. PMID: 20419147 Free PMC article.
-
Pdlim7 is required for maintenance of the mesenchymal/epidermal Fgf signaling feedback loop during zebrafish pectoral fin development.BMC Dev Biol. 2010 Oct 15;10:104. doi: 10.1186/1471-213X-10-104. BMC Dev Biol. 2010. PMID: 20950450 Free PMC article.
-
Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio).Int J Dev Biol. 2004;48(2-3):217-31. doi: 10.1387/ijdb.15272388. Int J Dev Biol. 2004. PMID: 15272388 Review.
-
Roles of Fibulin-2 in Carcinogenesis.Med Sci Monit. 2020 Jan 9;26:e918099. doi: 10.12659/MSM.918099. Med Sci Monit. 2020. PMID: 31915327 Free PMC article. Review.
Cited by
-
The role of adhesion protein Fibulin7 in development and diseases.Mol Med. 2020 May 19;26(1):47. doi: 10.1186/s10020-020-00169-z. Mol Med. 2020. PMID: 32429873 Free PMC article. Review.
-
Planarian EGF repeat-containing genes megf6 and hemicentin are required to restrict the stem cell compartment.PLoS Genet. 2020 Feb 20;16(2):e1008613. doi: 10.1371/journal.pgen.1008613. eCollection 2020 Feb. PLoS Genet. 2020. PMID: 32078629 Free PMC article.
-
A Hierarchy of Proliferative and Migratory Keratinocytes Maintains the Tympanic Membrane.Cell Stem Cell. 2021 Feb 4;28(2):315-330.e5. doi: 10.1016/j.stem.2020.10.006. Epub 2020 Nov 11. Cell Stem Cell. 2021. PMID: 33181078 Free PMC article.
-
Protein anabolism is key to long-term survival in high-grade serous ovarian cancer.Transl Oncol. 2021 Jan;14(1):100885. doi: 10.1016/j.tranon.2020.100885. Epub 2020 Oct 9. Transl Oncol. 2021. PMID: 33045680 Free PMC article.
-
Independent mesenchymal progenitor pools respectively produce and maintain osteogenic and chondrogenic cells in zebrafish.Dev Growth Differ. 2024 Feb;66(2):161-171. doi: 10.1111/dgd.12908. Epub 2024 Jan 9. Dev Growth Differ. 2024. PMID: 38193362 Free PMC article.
References
-
- Argraves WS, Dickerson K, Burgess WH, Ruoslahti E. Fibulin, a novel protein that interacts with the fibronectin receptor beta subunit cytoplasmic domain. Cell. 1989;58:623–629. - PubMed
-
- Balbona K, Tran H, Godyna S, Ingham KC, Strickland DK, Argraves WS. Fibulin binds to itself and to the carboxyl-terminal heparin-binding region of fibronectin. J Biol Chem. 1992;267:20120–20125. - PubMed
-
- Barth JL, Argraves KM, Roark EF, Little CD, Argraves WS. Identification of chicken and C. elegans fibulin-1 homologs and characterization of the C. elegans fibulin-1 gene. Matrix Biol. 1998;17:635–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

