Role of the inositol 1,4,5-trisphosphate receptor/Ca2+-release channel in autophagy
- PMID: 22770472
- PMCID: PMC3413604
- DOI: 10.1186/1478-811X-10-17
Role of the inositol 1,4,5-trisphosphate receptor/Ca2+-release channel in autophagy
Abstract
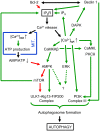
Autophagy is an important cell-biological process responsible for the disposal of long-lived proteins, protein aggregates, defective organelles and intracellular pathogens. It is activated in response to cellular stress and plays a role in development, cell differentiation, and ageing. Moreover, it has been shown to be involved in different pathologies, including cancer and neurodegenerative diseases. It is a long standing issue whether and how the Ca2+ ion is involved in its regulation. The role of the inositol 1,4,5-trisphosphate receptor, the main intracellular Ca2+-release channel, in apoptosis is well recognized, but its role in autophagy only recently emerged and is therefore much less well understood. Positive as well as negative effects on autophagy have been reported for both the inositol 1,4,5-trisphosphate receptor and Ca2+. This review will critically present the evidence for a role of the inositol 1,4,5-trisphosphate receptor/Ca2+-release channel in autophagy and will demonstrate that depending on the cellular conditions it can either suppress or promote autophagy. Suppression occurs through Ca2+ signals directed to the mitochondria, fueling ATP production and decreasing AMP-activated kinase activity. In contrast, Ca2+-induced autophagy can be mediated by several pathways including calmodulin-dependent kinase kinase β, calmodulin-dependent kinase I, protein kinase C θ, and/or extracellular signal-regulated kinase.
Figures
Similar articles
-
Transient release of calcium from inositol 1,4,5-trisphosphate-specific stores regulates mouse preimplantation development.Development. 1996 Aug;122(8):2485-96. doi: 10.1242/dev.122.8.2485. Development. 1996. PMID: 8756293
-
Protein kinase C modulates the insulin secretory process by maintaining a proper function of the beta-cell voltage-activated Ca2+ channels.J Biol Chem. 1994 Jan 28;269(4):2743-9. J Biol Chem. 1994. PMID: 8300606
-
Signaling Pathway for Endothelin-1- and Phenylephrine-Induced cAMP Response Element Binding Protein Activation in Rat Ventricular Myocytes: Role of Inositol 1,4,5-Trisphosphate Receptors and CaMKII.Cell Physiol Biochem. 2017;41(1):399-412. doi: 10.1159/000456422. Epub 2017 Jan 26. Cell Physiol Biochem. 2017. PMID: 28214885
-
Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation.Biochim Biophys Acta. 2009 Jun;1793(6):959-70. doi: 10.1016/j.bbamcr.2008.12.003. Epub 2008 Dec 16. Biochim Biophys Acta. 2009. PMID: 19133301 Free PMC article. Review.
-
Regulation and Role of Store-Operated Ca2+ Entry in Cellular Proliferation.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 12. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 12. PMID: 30299656 Free Books & Documents. Review.
Cited by
-
Conditional Loss of Hoxa5 Function Early after Birth Impacts on Expression of Genes with Synaptic Function.Front Mol Neurosci. 2017 Nov 15;10:369. doi: 10.3389/fnmol.2017.00369. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29187810 Free PMC article.
-
The sigma-1 receptor: a regulator of cancer cell electrical plasticity?Front Physiol. 2013 Jul 16;4:175. doi: 10.3389/fphys.2013.00175. eCollection 2013. Front Physiol. 2013. PMID: 23882221 Free PMC article.
-
Dysregulation of the miR-25-IMPA2 axis promotes metastatic progression in clear cell renal cell carcinoma.EBioMedicine. 2019 Jul;45:220-230. doi: 10.1016/j.ebiom.2019.06.006. Epub 2019 Jun 12. EBioMedicine. 2019. PMID: 31202813 Free PMC article.
-
PLCγ1 inhibition-driven autophagy of IL-1β-treated chondrocyte confers cartilage protection against osteoarthritis, involving AMPK, Erk and Akt.J Cell Mol Med. 2021 Feb;25(3):1531-1545. doi: 10.1111/jcmm.16245. Epub 2020 Dec 28. J Cell Mol Med. 2021. PMID: 33372388 Free PMC article.
-
NAADP-mediated Ca2+ signaling promotes autophagy and protects against LPS-induced liver injury.FASEB J. 2017 Jul;31(7):3126-3137. doi: 10.1096/fj.201601290R. Epub 2017 Apr 6. FASEB J. 2017. PMID: 28386045 Free PMC article.
References
-
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqui FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. - PubMed
-
- Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263:55–72. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

