In vitro-in vivo translation of lipid nanoparticles for hepatocellular siRNA delivery
- PMID: 22770391
- PMCID: PMC3429655
- DOI: 10.1021/nn301922x
In vitro-in vivo translation of lipid nanoparticles for hepatocellular siRNA delivery
Abstract
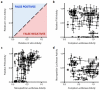
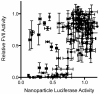
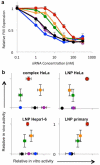
A significant challenge in the development of clinically viable siRNA delivery systems is a lack of in vitro-in vivo translatability: many delivery vehicles that are initially promising in cell culture do not retain efficacy in animals. Despite its importance, little information exists on the predictive nature of in vitro methodologies, most likely due to the cost and time associated with generating in vitro-in vivo data sets. Recently, high-throughput techniques have been developed that have allowed the examination of hundreds of lipid nanoparticle formulations for transfection efficiency in multiple experimental systems. The large resulting data set has allowed the development of correlations between in vitro and characterization data and in vivo efficacy for hepatocellular delivery vehicles. Consistency of formulation technique and the type of cell used for in vitro experiments was found to significantly affect correlations, with primary hepatocytes and HeLa cells yielding the most predictive data. Interestingly, in vitro data acquired using HeLa cells were more predictive of in vivo performance than mouse hepatoma Hepa1-6 cells. Of the characterization parameters, only siRNA entrapment efficiency was partially predictive of in vivo silencing potential, while zeta-potential and, surprisingly, nanoparticle size (when <300 nm) as measured by dynamic light scattering were not. These data provide guiding principles in the development of clinically viable siRNA delivery materials and have the potential to reduce experimental costs while improving the translation of materials into animals.
Figures
Similar articles
-
Low molecular weight chitosan conjugated with folate for siRNA delivery in vitro: optimization studies.Int J Nanomedicine. 2012;7:5833-45. doi: 10.2147/IJN.S35567. Epub 2012 Nov 23. Int J Nanomedicine. 2012. PMID: 23209368 Free PMC article.
-
Efficient in vitro gene therapy with PEG siRNA lipid nanocapsules for passive targeting strategy in melanoma.Biotechnol J. 2014 Nov;9(11):1389-401. doi: 10.1002/biot.201400162. Epub 2014 Oct 18. Biotechnol J. 2014. PMID: 25262914
-
Nanometer-scale siRNA carriers incorporating peptidomimetic oligomers: physical characterization and biological activity.Int J Nanomedicine. 2014 May 10;9:2271-85. doi: 10.2147/IJN.S57449. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24872690 Free PMC article.
-
Enhancing endosomal escape for nanoparticle mediated siRNA delivery.Nanoscale. 2014 Jun 21;6(12):6415-25. doi: 10.1039/c4nr00018h. Nanoscale. 2014. PMID: 24837409 Review.
-
Lipid-based nanotherapeutics for siRNA delivery.J Intern Med. 2010 Jan;267(1):9-21. doi: 10.1111/j.1365-2796.2009.02189.x. J Intern Med. 2010. PMID: 20059641 Free PMC article. Review.
Cited by
-
Kinetic Modeling to Accelerate the Development of Nucleic Acid Formulations.ACS Nano. 2021 Oct 26;15(10):16055-16066. doi: 10.1021/acsnano.1c04555. Epub 2021 Oct 12. ACS Nano. 2021. PMID: 34636541 Free PMC article.
-
Current Progress on MicroRNA-Based Gene Delivery in the Treatment of Osteoporosis and Osteoporotic Fracture.Int J Endocrinol. 2019 Mar 6;2019:6782653. doi: 10.1155/2019/6782653. eCollection 2019. Int J Endocrinol. 2019. PMID: 30962808 Free PMC article. Review.
-
Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells.Nano Lett. 2018 Oct 10;18(10):6449-6454. doi: 10.1021/acs.nanolett.8b02917. Epub 2018 Sep 20. Nano Lett. 2018. PMID: 30211557 Free PMC article.
-
Let's get small (and smaller): Combining zebrafish and nanomedicine to advance neuroregenerative therapeutics.Adv Drug Deliv Rev. 2019 Aug;148:344-359. doi: 10.1016/j.addr.2019.01.011. Epub 2019 Feb 12. Adv Drug Deliv Rev. 2019. PMID: 30769046 Free PMC article. Review.
-
A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation.Nano Lett. 2018 Mar 14;18(3):2148-2157. doi: 10.1021/acs.nanolett.8b00432. Epub 2018 Mar 5. Nano Lett. 2018. PMID: 29489381 Free PMC article.
References
-
- Yamada KM, Cukierman E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell. 2007;130:601–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

