Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation
- PMID: 22769591
- PMCID: PMC3477299
- DOI: 10.1021/cb3002599
Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation
Abstract
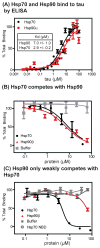
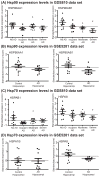
The microtubule associated protein tau (MAPT/tau) aberrantly accumulates in 15 neurodegenerative diseases, termed tauopathies. One way to treat tauopathies may be to accelerate tau clearance, but the molecular mechanisms governing tau stability are not yet clear. We recently identified chemical probes that markedly accelerate the clearance of tau in cellular and animal models. In the current study, we used one of these probes in combination with immunoprecipitation and mass spectrometry to identify 48 proteins whose association with tau changes during the first 10 min after treatment. These proteins included known modifiers of tau proteotoxicity, such as ILF-2 (NFAT), ILF-3, and ataxin-2. A striking observation from the data set was that tau binding to heat shock protein 70 (Hsp70) decreased, whereas binding to Hsp90 significantly increased. Both chaperones have been linked to tau homeostasis, but their mechanisms have not been established. Using peptide arrays and binding assays, we found that Hsp70 and Hsp90 appeared to compete for binding to shared sites on tau. Further, the Hsp90-bound complex proved to be important in initiating tau clearance in cells. These results suggest that the relative levels of Hsp70 and Hsp90 may help determine whether tau is retained or degraded. Consistent with this model, analysis of reported microarray expression data from Alzheimer's disease patients and age-matched controls showed that the levels of Hsp90 are reduced in the diseased hippocampus. These studies suggest that Hsp70 and Hsp90 work together to coordinate tau homeostasis.
Figures


Similar articles
-
Hsp multichaperone complex buffers pathologically modified Tau.Nat Commun. 2022 Jun 27;13(1):3668. doi: 10.1038/s41467-022-31396-z. Nat Commun. 2022. PMID: 35760815 Free PMC article.
-
The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins.J Clin Invest. 2007 Mar;117(3):648-58. doi: 10.1172/JCI29715. Epub 2007 Feb 15. J Clin Invest. 2007. PMID: 17304350 Free PMC article.
-
Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies.Future Med Chem. 2011 Sep;3(12):1523-37. doi: 10.4155/fmc.11.88. Future Med Chem. 2011. PMID: 21882945 Free PMC article. Review.
-
Stabilizing the Hsp70-Tau Complex Promotes Turnover in Models of Tauopathy.Cell Chem Biol. 2016 Aug 18;23(8):992-1001. doi: 10.1016/j.chembiol.2016.04.014. Epub 2016 Aug 4. Cell Chem Biol. 2016. PMID: 27499529 Free PMC article.
-
Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer's disease.Prog Neurobiol. 2011 Jan;93(1):99-110. doi: 10.1016/j.pneurobio.2010.10.006. Epub 2010 Nov 5. Prog Neurobiol. 2011. PMID: 21056617 Review.
Cited by
-
Accelerated neurodegeneration through chaperone-mediated oligomerization of tau.J Clin Invest. 2013 Oct;123(10):4158-69. doi: 10.1172/JCI69003. Epub 2013 Sep 3. J Clin Invest. 2013. PMID: 23999428 Free PMC article.
-
The mechanism of Hsp90-induced oligomerizaton of Tau.Sci Adv. 2020 Mar 13;6(11):eaax6999. doi: 10.1126/sciadv.aax6999. eCollection 2020 Mar. Sci Adv. 2020. PMID: 32201713 Free PMC article.
-
BAG3 Is a Modular, Scaffolding Protein that physically Links Heat Shock Protein 70 (Hsp70) to the Small Heat Shock Proteins.J Mol Biol. 2017 Jan 6;429(1):128-141. doi: 10.1016/j.jmb.2016.11.013. Epub 2016 Nov 21. J Mol Biol. 2017. PMID: 27884606 Free PMC article.
-
Temperature-induced Artifacts in Tau Phosphorylation: Implications for Reliable Alzheimer's Disease Research.Exp Neurobiol. 2023 Dec 31;32(6):423-440. doi: 10.5607/en23025. Exp Neurobiol. 2023. PMID: 38196137 Free PMC article.
-
Hsp multichaperone complex buffers pathologically modified Tau.Nat Commun. 2022 Jun 27;13(1):3668. doi: 10.1038/s41467-022-31396-z. Nat Commun. 2022. PMID: 35760815 Free PMC article.
References
-
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. - PubMed
-
- Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

