Interplay of Nkx3.2, Sox9 and Pax3 regulates chondrogenic differentiation of muscle progenitor cells
- PMID: 22768305
- PMCID: PMC3388093
- DOI: 10.1371/journal.pone.0039642
Interplay of Nkx3.2, Sox9 and Pax3 regulates chondrogenic differentiation of muscle progenitor cells
Abstract
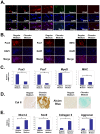
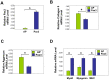
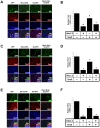
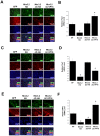
Muscle satellite cells make up a stem cell population that is capable of differentiating into myocytes and contributing to muscle regeneration upon injury. In this work we investigate the mechanism by which these muscle progenitor cells adopt an alternative cell fate, the cartilage fate. We show that chick muscle satellite cells that normally would undergo myogenesis can be converted to express cartilage matrix proteins in vitro when cultured in chondrogenic medium containing TGFß3 or BMP2. In the meantime, the myogenic program is repressed, suggesting that muscle satellite cells have undergone chondrogenic differentiation. Furthermore, ectopic expression of the myogenic factor Pax3 prevents chondrogenesis in these cells, while chondrogenic factors Nkx3.2 and Sox9 act downstream of TGFß or BMP2 to promote this cell fate transition. We found that Nkx3.2 and Sox9 repress the activity of the Pax3 promoter and that Nkx3.2 acts as a transcriptional repressor in this process. Importantly, a reverse function mutant of Nkx3.2 blocks the ability of Sox9 to both inhibit myogenesis and induce chondrogenesis, suggesting that Nkx3.2 is required for Sox9 to promote chondrogenic differentiation in satellite cells. Finally, we found that in an in vivo mouse model of fracture healing where muscle progenitor cells were lineage-traced, Nkx3.2 and Sox9 are significantly upregulated while Pax3 is significantly downregulated in the muscle progenitor cells that give rise to chondrocytes during fracture repair. Thus our in vitro and in vivo analyses suggest that the balance of Pax3, Nkx3.2 and Sox9 may act as a molecular switch during the chondrogenic differentiation of muscle progenitor cells, which may be important for fracture healing.
Conflict of interest statement
Figures
Similar articles
-
Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis.Genes Dev. 2002 Aug 1;16(15):1990-2005. doi: 10.1101/gad.1008002. Genes Dev. 2002. PMID: 12154128 Free PMC article.
-
A gradient of Shh establishes mutually repressing somitic cell fates induced by Nkx3.2 and Pax3.Dev Biol. 2008 Nov 15;323(2):152-65. doi: 10.1016/j.ydbio.2008.08.024. Epub 2008 Sep 5. Dev Biol. 2008. PMID: 18796301 Free PMC article.
-
Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation.PLoS One. 2014 Feb 13;9(2):e89025. doi: 10.1371/journal.pone.0089025. eCollection 2014. PLoS One. 2014. PMID: 24551211 Free PMC article.
-
Satellite cells, the engines of muscle repair.Nat Rev Mol Cell Biol. 2011 Dec 21;13(2):127-33. doi: 10.1038/nrm3265. Nat Rev Mol Cell Biol. 2011. PMID: 22186952 Review.
-
Regulation and function of SOX9 during cartilage development and regeneration.Semin Cancer Biol. 2020 Dec;67(Pt 1):12-23. doi: 10.1016/j.semcancer.2020.04.008. Epub 2020 May 4. Semin Cancer Biol. 2020. PMID: 32380234 Review.
Cited by
-
VAV3 Overexpressed in Cancer Stem Cells Is a Poor Prognostic Indicator in Ovarian Cancer Patients.Stem Cells Dev. 2015 Jul 1;24(13):1521-35. doi: 10.1089/scd.2014.0588. Epub 2015 Apr 9. Stem Cells Dev. 2015. PMID: 25715123 Free PMC article.
-
The role of muscle in bone repair: the cells, signals, and tissue responses to injury.Curr Osteoporos Rep. 2013 Jun;11(2):130-5. doi: 10.1007/s11914-013-0146-3. Curr Osteoporos Rep. 2013. PMID: 23591779 Free PMC article. Review.
-
Molecular mechanisms of heterotopic ossification.J Hand Surg Am. 2014 Mar;39(3):563-6. doi: 10.1016/j.jhsa.2013.09.029. Epub 2013 Nov 15. J Hand Surg Am. 2014. PMID: 24246757 Free PMC article. Review. No abstract available.
-
Identification of satellite cells from anole lizard skeletal muscle and demonstration of expanded musculoskeletal potential.Dev Biol. 2018 Jan 15;433(2):344-356. doi: 10.1016/j.ydbio.2017.08.037. Epub 2017 Dec 25. Dev Biol. 2018. PMID: 29291980 Free PMC article.
-
Inhibition of miR-101-3p prevents human aortic valve interstitial cell calcification through regulation of CDH11/SOX9 expression.Mol Med. 2023 Feb 21;29(1):24. doi: 10.1186/s10020-023-00619-4. Mol Med. 2023. PMID: 36809926 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

