T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair
- PMID: 22768215
- PMCID: PMC3386936
- DOI: 10.1371/journal.pone.0040044
T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair
Abstract
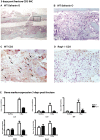
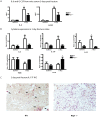
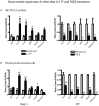
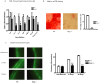
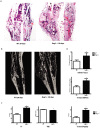
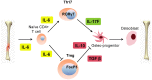
While it is well known that the presence of lymphocytes and cytokines are important for fracture healing, the exact role of the various cytokines expressed by cells of the immune system on osteoblast biology remains unclear. To study the role of inflammatory cytokines in fracture repair, we studied tibial bone healing in wild-type and Rag1(-/-) mice. Histological analysis, µCT stereology, biomechanical testing, calcein staining and quantitative RNA gene expression studies were performed on healing tibial fractures. These data provide support for Rag1(-/-) mice as a model of impaired fracture healing compared to wild-type. Moreover, the pro-inflammatory cytokine, IL-17F, was found to be a key mediator in the cellular response of the immune system in osteogenesis. In vitro studies showed that IL-17F alone stimulated osteoblast maturation. We propose a model in which the Th17 subset of T-lymphocytes produces IL-17F to stimulate bone healing. This is a pivotal link in advancing our current understanding of the molecular and cellular basis of fracture healing, which in turn may aid in optimizing fracture management and in the treatment of impaired bone healing.
Conflict of interest statement
Figures
Similar articles
-
Fracture healing is accelerated in the absence of the adaptive immune system.J Bone Miner Res. 2011 Jan;26(1):113-24. doi: 10.1002/jbmr.185. J Bone Miner Res. 2011. PMID: 20641004
-
A two phase regulation of bone regeneration: IL-17F mediates osteoblastogenesis via C/EBP-β in vitro.Bone. 2018 Nov;116:47-57. doi: 10.1016/j.bone.2018.07.007. Epub 2018 Jul 17. Bone. 2018. PMID: 30010083
-
MiR-142-5p promotes bone repair by maintaining osteoblast activity.J Bone Miner Metab. 2017 May;35(3):255-264. doi: 10.1007/s00774-016-0757-8. Epub 2016 Apr 16. J Bone Miner Metab. 2017. PMID: 27085967
-
Osteoimmunology in Bone Fracture Healing.Curr Osteoporos Rep. 2017 Aug;15(4):367-375. doi: 10.1007/s11914-017-0381-0. Curr Osteoporos Rep. 2017. PMID: 28647888 Review.
-
Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation.J Cell Biochem. 2003 Apr 1;88(5):873-84. doi: 10.1002/jcb.10435. J Cell Biochem. 2003. PMID: 12616527 Review.
Cited by
-
Immature myeloid cells are critical for enhancing bone fracture healing through angiogenic cascade.Bone. 2016 Dec;93:113-124. doi: 10.1016/j.bone.2016.09.018. Epub 2016 Sep 21. Bone. 2016. PMID: 27664567 Free PMC article.
-
Isolated metaphyseal injury influences unrelated bones.Acta Orthop. 2017 Apr;88(2):223-230. doi: 10.1080/17453674.2016.1274587. Epub 2017 Jan 27. Acta Orthop. 2017. PMID: 28128005 Free PMC article.
-
Bioinformatics Analysis of the Key Genes and Pathways in Multiple Myeloma.Int J Gen Med. 2022 Sep 5;15:6999-7016. doi: 10.2147/IJGM.S377321. eCollection 2022. Int J Gen Med. 2022. PMID: 36090706 Free PMC article.
-
An exploration of the causal relationship between 731 immunophenotypes and osteoporosis: a bidirectional Mendelian randomized study.Front Endocrinol (Lausanne). 2024 Jul 17;15:1341002. doi: 10.3389/fendo.2024.1341002. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39086903 Free PMC article.
-
Bone Involvement in Patients with Spondyloarthropathies.Calcif Tissue Int. 2022 Apr;110(4):393-420. doi: 10.1007/s00223-021-00933-1. Epub 2022 Jan 23. Calcif Tissue Int. 2022. PMID: 35066596 Review.
References
-
- Gerstenfeld LC, Cho T-J, Kon T, Aizawa T, Tsay A, et al. Impaired Fracture Healing in the Absence of TNF-α Signaling: The Role of TNF-α in Endochondral Cartilage Resorption. J Bone Miner Res. 2003;18:1584–1592. - PubMed
-
- Andrew JG, Andrew SM, Freemont AJ, Marsh DR. Inflammatory cells in normal human fracture healing. Acta Orthop Scand. 1994;65:462–6. - PubMed
-
- Cho T-J, Gerstenfeld LC, Barnes GL, Einhorn T. Cytokines and fracture healing. Current Opin Orthop. 2001;12:403–408.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

