Vessel-associated transforming growth factor-beta1 (TGF-β1) is increased in the bronchial reticular basement membrane in COPD and normal smokers
- PMID: 22768115
- PMCID: PMC3387255
- DOI: 10.1371/journal.pone.0039736
Vessel-associated transforming growth factor-beta1 (TGF-β1) is increased in the bronchial reticular basement membrane in COPD and normal smokers
Abstract
Background: Transforming growth factor-beta1 (TGF-β1) is a multipotential cytokine with angiogenic activity. There are only limited data about its role in airway remodeling in COPD. We have previously shown that the reticular basement membrane (Rbm) is hypervascular in the airways of current smokers either with or without chronic obstructive pulmonary disease (COPD). This study evaluated TGF-β1 immunostaining in the Rbm and its relationship to vascularity in smokers with or without COPD.
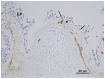
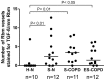
Methodology/principal findings: Bronchial biopsies from 15 smokers with normal lung function, 19 current and 14 ex-smokers with COPD were immunostained for TGF-β1 antibody and compared to 17 healthy controls. The percentage area of tissue and also number and area of vessels staining positively for TGF-β1 were measured and compared between groups. Some bronchial biopsies from current smoking COPD subjects were also stained for phosphorylated (active) Smad2/3. Epithelial TGF- β1 staining was not different between COPD current smokers and normal controls. TGF-β1 stained vessels in the Rbm were increased in smokers with normal lung function, current smoking COPD and ex-smokers with COPD compared to controls [median (range) for number of vessels/mm Rbm 2.5 (0.0-12.7), 3.4 (0.0-8.1) and 1.0 (0.0-6.3) vs. 0.0 (0.0-7.0), p<0.05]. Percentage of vessels stained was also increased in these clinical groups. Preliminary data suggest that in current smoking COPD subjects endothelial cells and cells in the Rbm stain positively for phosphorylated Smad2/3 suggesting TGF-β1 is functionally active in this situation.
Conclusions/significance: Vessel-associated TGF-β1 activity is increased in the bronchial Rbm in smokers and especially those with COPD.
Conflict of interest statement
Figures


Similar articles
-
Basement membrane and vascular remodelling in smokers and chronic obstructive pulmonary disease: a cross-sectional study.Respir Res. 2010 Jul 30;11(1):105. doi: 10.1186/1465-9921-11-105. Respir Res. 2010. PMID: 20670454 Free PMC article.
-
Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease.Respirology. 2010 Aug;15(6):930-8. doi: 10.1111/j.1440-1843.2010.01808.x. Epub 2010 Jul 12. Respirology. 2010. PMID: 20630030
-
Transforming growth factor (TGF) β1 and Smad signalling pathways: A likely key to EMT-associated COPD pathogenesis.Respirology. 2017 Jan;22(1):133-140. doi: 10.1111/resp.12882. Epub 2016 Sep 11. Respirology. 2017. PMID: 27614607
-
Transforming growth factor-β1 and SMAD signalling pathway in the small airways of smokers and patients with COPD: potential role in driving fibrotic type-2 epithelial mesenchymal transition.Front Immunol. 2023 Jun 26;14:1216506. doi: 10.3389/fimmu.2023.1216506. eCollection 2023. Front Immunol. 2023. PMID: 37435075 Free PMC article.
-
Remodeling in asthma and chronic obstructive lung disease.Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 2):S28-38. doi: 10.1164/ajrccm.164.supplement_2.2106061. Am J Respir Crit Care Med. 2001. PMID: 11734464 Review.
Cited by
-
Recuperating lung decoction attenuates inflammation and oxidation in cigarette smoke-induced COPD in rats via activation of ERK and Nrf2 pathways.Cell Biochem Funct. 2017 Jul;35(5):278-286. doi: 10.1002/cbf.3273. Cell Biochem Funct. 2017. PMID: 28749079 Free PMC article.
-
Endothelial to mesenchymal transition is an active process in smokers and patients with early COPD contributing to pulmonary arterial pathology.ERJ Open Res. 2024 Feb 12;10(1):00767-2023. doi: 10.1183/23120541.00767-2023. eCollection 2024 Jan. ERJ Open Res. 2024. PMID: 38348240 Free PMC article.
-
Effects of Chung-Pae Inhalation Therapy on a Mouse Model of Chronic Obstructive Pulmonary Disease.Evid Based Complement Alternat Med. 2015;2015:461295. doi: 10.1155/2015/461295. Epub 2015 Oct 11. Evid Based Complement Alternat Med. 2015. PMID: 26539225 Free PMC article.
-
Transforming Growth Factor-β1 Increases DNA Methyltransferase 1 and 3a Expression through Distinct Post-transcriptional Mechanisms in Lung Fibroblasts.J Biol Chem. 2016 Sep 9;291(37):19287-98. doi: 10.1074/jbc.M116.723080. Epub 2016 Jul 12. J Biol Chem. 2016. PMID: 27405758 Free PMC article.
-
Fully integrating pathophysiological insights in COPD: an updated working disease model to broaden therapeutic vision.Eur Respir Rev. 2021 May 25;30(160):200364. doi: 10.1183/16000617.0364-2020. Print 2021 Jun 30. Eur Respir Rev. 2021. PMID: 34039673 Free PMC article. Review.
References
-
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. - PubMed
-
- Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD Prevalence Estimates: What Is the True Burden of Disease? Chest. 2003;123:1684–1692. - PubMed
-
- Hogg JC. Lung structure and function in COPD. Int J Tuberc Lung Dis. 2008;12:467–479. - PubMed
-
- GOLD. Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the Diagnosis, Mangement and Prevention of Chronic Obstructive Pulmonary Disease Updated 2009. 2009. Available: http://wwwgoldcopdorg/guidelines-global-strategy-for-diagnosis-managemen....
-
- Boulet LP, Sterk PJ. Airway remodelling: the future. Eur Respir J. 2007;30:831–834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

