7-Substituted 8-aza-7-deazaadenosines for modification of the siRNA major groove
- PMID: 22766576
- PMCID: PMC3417767
- DOI: 10.1039/c2ob25647a
7-Substituted 8-aza-7-deazaadenosines for modification of the siRNA major groove
Abstract
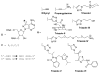
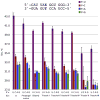
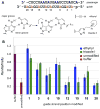
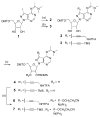
Here we describe the synthesis of new 7-substituted 8-aza-7-deazaadenosine ribonucleoside phosphoramidites and their use in generating major groove-modified duplex RNAs. A 7-ethynyl analog leads to further structural diversification of the RNA via post-automated RNA synthesis azide-alkyne cycloaddition reactions. In addition, we report preliminary studies on the effects of eight different purine 7-position modifications on RNA duplex stability and pairing specificity. Finally, the effect on RNAi activity of this type of modification at eight different positions in an siRNA guide strand has been explored. Analogs were identified with large 7-position substituents that maintain adenosine pairing specificity and are well-tolerated at specific positions in an siRNA guide strand.
Figures
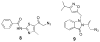
Similar articles
-
Click modification of RNA at adenosine: structure and reactivity of 7-ethynyl- and 7-triazolyl-8-aza-7-deazaadenosine in RNA.ACS Chem Biol. 2014 Aug 15;9(8):1780-7. doi: 10.1021/cb500270x. Epub 2014 Jun 16. ACS Chem Biol. 2014. PMID: 24896732 Free PMC article.
-
N(2)-Modified 2-aminopurine ribonucleosides as minor-groove-modulating adenosine replacements in duplex RNA.Org Lett. 2010 Mar 5;12(5):1044-7. doi: 10.1021/ol100019r. Org Lett. 2010. PMID: 20108910 Free PMC article.
-
Modification of the siRNA passenger strand by 5-nitroindole dramatically reduces its off-target effects.Chembiochem. 2012 Sep 3;13(13):1940-5. doi: 10.1002/cbic.201200349. Epub 2012 Aug 7. Chembiochem. 2012. PMID: 22887813
-
Preparation of 5'-silyl-2'-orthoester ribonucleosides for use in oligoribonucleotide synthesis.Curr Protoc Nucleic Acid Chem. 2004 May;Chapter 2:Unit 2.10. doi: 10.1002/0471142700.nc0210s16. Curr Protoc Nucleic Acid Chem. 2004. PMID: 18428924 Review.
-
[Chemical Approaches for RNAi Drug Development].Yakugaku Zasshi. 2020;140(10):1259-1268. doi: 10.1248/yakushi.20-00157. Yakugaku Zasshi. 2020. PMID: 32999205 Review. Japanese.
Cited by
-
Click modification of RNA at adenosine: structure and reactivity of 7-ethynyl- and 7-triazolyl-8-aza-7-deazaadenosine in RNA.ACS Chem Biol. 2014 Aug 15;9(8):1780-7. doi: 10.1021/cb500270x. Epub 2014 Jun 16. ACS Chem Biol. 2014. PMID: 24896732 Free PMC article.
-
Synthesis and evaluation of an alkyne-modified ATP analog for enzymatic incorporation into RNA.Bioorg Med Chem Lett. 2016 Apr 1;26(7):1799-802. doi: 10.1016/j.bmcl.2016.02.038. Epub 2016 Feb 18. Bioorg Med Chem Lett. 2016. PMID: 26927424 Free PMC article.
-
Promiscuous 8-alkoxyadenosines in the guide strand of an siRNA: modulation of silencing efficacy and off-pathway protein binding.J Am Chem Soc. 2012 Oct 24;134(42):17643-52. doi: 10.1021/ja307102g. Epub 2012 Oct 11. J Am Chem Soc. 2012. PMID: 23030736 Free PMC article.
-
Controlling miRNA-like off-target effects of an siRNA with nucleobase modifications.Org Biomol Chem. 2017 Dec 6;15(47):10029-10036. doi: 10.1039/c7ob02654d. Org Biomol Chem. 2017. PMID: 29164215 Free PMC article.
-
Synthesis of Nucleobase-Modified RNA Oligonucleotides by Post-Synthetic Approach.Molecules. 2020 Jul 23;25(15):3344. doi: 10.3390/molecules25153344. Molecules. 2020. PMID: 32717917 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

