Role of c-Myb in the survival of pre B-cell acute lymphoblastic leukemia and leukemogenesis
- PMID: 22764095
- PMCID: PMC3578739
- DOI: 10.1002/ajh.23283
Role of c-Myb in the survival of pre B-cell acute lymphoblastic leukemia and leukemogenesis
Abstract
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. The current treatment protocol for ALL involves an intense chemotherapy regimen yielding cure rates of nearly 80%. However, new therapies need to be designed not only to increase the survival rate but also to combat the risk of severe therapy associated toxicities including secondary malignancies, growth problems, organ damage, and infertility. The c-Myb proto-oncogene is highly expressed in immature hematopoietic cells. In this study, we demonstrate that loss of c-Myb itself decreased the viability of these leukemic cells. Additionally, the inhibition of c-Myb caused a decrease in cell proliferation, significantly increased the number of cells in G(0) /G(1) phase of the cell cycle, increased the sensitivity of pre-B-ALL cells to cytotoxic agents in vitro, and significantly delayed disease onset in a mouse model of leukemia. Furthermore, we demonstrate that Bcl-2 is a target of c-Myb in pre-B-ALL cells. Our results identify c-Myb as a potential therapeutic target in pre-B-ALL and suggest that suppression of c-Myb levels or activity, in combination with currently used therapies and/or dose reduction, may lead to a decrease in toxicity and an increase in patient survival rates. Because c-Myb is aberrantly expressed in several other malignancies, targeting c-Myb will have broad clinical applications.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Nothing to report.
Figures
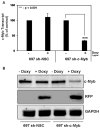

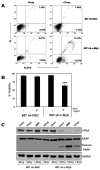
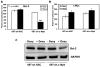
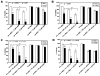
Similar articles
-
Mer receptor tyrosine kinase is a novel therapeutic target in pediatric B-cell acute lymphoblastic leukemia.Blood. 2009 Sep 24;114(13):2678-87. doi: 10.1182/blood-2009-03-209247. Epub 2009 Jul 30. Blood. 2009. Retraction in: Blood. 2012 Aug 16;120(7):1533. doi: 10.1182/blood-2012-06-440792 PMID: 19643988 Free PMC article. Retracted.
-
c-Myb interacts with the glucocorticoid receptor and regulates its level in pre-B-acute lymphoblastic leukemia cells.Mol Cell Endocrinol. 2012 Sep 25;361(1-2):124-32. doi: 10.1016/j.mce.2012.03.024. Epub 2012 Apr 10. Mol Cell Endocrinol. 2012. PMID: 22516378 Free PMC article.
-
Mer receptor tyrosine kinase is a therapeutic target in pre-B-cell acute lymphoblastic leukemia.Blood. 2013 Aug 29;122(9):1599-609. doi: 10.1182/blood-2013-01-478156. Epub 2013 Jul 16. Blood. 2013. PMID: 23861246 Free PMC article.
-
Targeting c-Myb expression in human disease.Expert Opin Ther Targets. 2003 Apr;7(2):235-48. doi: 10.1517/14728222.7.2.235. Expert Opin Ther Targets. 2003. PMID: 12667100 Review.
-
C-Myb function in the vessel wall.Front Biosci (Elite Ed). 2011 Jun 1;3(3):968-77. doi: 10.2741/e302. Front Biosci (Elite Ed). 2011. PMID: 21622105 Review.
Cited by
-
Reassessing the Potential of Myb-targeted Anti-cancer Therapy.J Cancer. 2018 Mar 15;9(7):1259-1266. doi: 10.7150/jca.23992. eCollection 2018. J Cancer. 2018. PMID: 29675107 Free PMC article. Review.
-
Overexpression of c-Myb is associated with suppression of distant metastases in colorectal carcinoma.Tumour Biol. 2016 Aug;37(8):10723-9. doi: 10.1007/s13277-016-4956-7. Epub 2016 Feb 12. Tumour Biol. 2016. PMID: 26873484
-
Epigenetic alteration by DNA-demethylating treatment restores apoptotic response to glucocorticoids in dexamethasone-resistant human malignant lymphoid cells.Cancer Cell Int. 2014 Apr 23;14:35. doi: 10.1186/1475-2867-14-35. eCollection 2014. Cancer Cell Int. 2014. PMID: 24795534 Free PMC article.
-
Emerging role of MYB transcription factors in cancer drug resistance.Cancer Drug Resist. 2024 Apr 30;7:15. doi: 10.20517/cdr.2023.158. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38835346 Free PMC article. Review.
-
Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: Molecular pathways and disease treatments.Oncol Lett. 2020 Jul;20(1):448-454. doi: 10.3892/ol.2020.11583. Epub 2020 May 4. Oncol Lett. 2020. PMID: 32565969 Free PMC article. Review.
References
-
- Pui CH. Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2010;109:777–787. - PubMed
-
- Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. - PubMed
-
- Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–2195. - PubMed
-
- Pui CH. Childhood Leukemias. Cambridge: Cambridge University Press; 2006. pp. 21–47.
-
- Raimondi SC, Behm FG, Roberson PK, et al. Cytogenetics of pre-B-cell acute lymphoblastic leukemia with emphasis on prognostic implications of the t(1;19) J Clin Oncol. 1990;8:1380–1388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
