Innate immune control of HIV
- PMID: 22762020
- PMCID: PMC3385945
- DOI: 10.1101/cshperspect.a007070
Innate immune control of HIV
Abstract
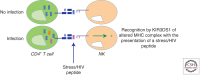
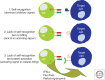
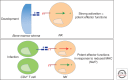
Mounting evidence suggests a role for innate immunity in the early control of HIV infection, before the induction of adaptive immune responses. Among the early innate immune effector cells, dendritic cells (DCs) respond rapidly following infection aimed at arming the immune system, through the recognition of viral products via pattern recognition receptors. This early response results in the potent induction of a cascade of inflammatory cytokines, intimately involved in directly setting up an antiviral state, and indirectly activating other antiviral cells of the innate immune system. However, epidemiologic data strongly support a role for natural killer (NK) cells as critical innate mediators of antiviral control, through the recognition of virally infected cells through a network of receptors called the killer immunoglobulin-like receptors (KIRs). In this review, the early events in innate immune recognition of HIV, focused on defining the biology underlying KIR-mediated NK-cell control of HIV viral replication, are discussed.
Figures
Similar articles
-
HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection.J Virol. 2009 Jul;83(13):6798-805. doi: 10.1128/JVI.00256-09. Epub 2009 Apr 22. J Virol. 2009. PMID: 19386717 Free PMC article.
-
KIR-HLA intercourse in HIV disease.Trends Microbiol. 2008 Dec;16(12):620-7. doi: 10.1016/j.tim.2008.09.002. Epub 2008 Oct 29. Trends Microbiol. 2008. PMID: 18976921 Free PMC article. Review.
-
Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers.AIDS. 2012 Sep 24;26(15):1869-78. doi: 10.1097/QAD.0b013e32835861b0. AIDS. 2012. PMID: 22874514 Free PMC article.
-
Peptide-Dependent Recognition of HLA-B*57:01 by KIR3DS1.J Virol. 2015 May;89(10):5213-21. doi: 10.1128/JVI.03586-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740999 Free PMC article.
-
KIR/HLA: genetic clues for a role of NK cells in the control of HIV.Adv Exp Med Biol. 2011;780:27-36. doi: 10.1007/978-1-4419-5632-3_3. Adv Exp Med Biol. 2011. PMID: 21842362 Review.
Cited by
-
Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression.Nature. 2014 Jul 31;511(7511):601-5. doi: 10.1038/nature13554. Epub 2014 Jul 9. Nature. 2014. PMID: 25043006 Free PMC article.
-
Selection of an HLA-C*03:04-Restricted HIV-1 p24 Gag Sequence Variant Is Associated with Viral Escape from KIR2DL3+ Natural Killer Cells: Data from an Observational Cohort in South Africa.PLoS Med. 2015 Nov 17;12(11):e1001900; discussion e1001900. doi: 10.1371/journal.pmed.1001900. eCollection 2015 Nov. PLoS Med. 2015. PMID: 26575988 Free PMC article.
-
Visualisation of Host-Pathogen Communication.Adv Exp Med Biol. 2023;1406:19-39. doi: 10.1007/978-3-031-26462-7_2. Adv Exp Med Biol. 2023. PMID: 37016109
-
Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease.Immunity. 2016 May 17;44(5):1052-68. doi: 10.1016/j.immuni.2016.04.022. Immunity. 2016. PMID: 27192569 Free PMC article. Review.
-
High Activation of γδ T Cells and the γδ2pos T-Cell Subset Is Associated With the Onset of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome, ANRS 12153 CAPRI NK.Front Immunol. 2019 Aug 27;10:2018. doi: 10.3389/fimmu.2019.02018. eCollection 2019. Front Immunol. 2019. PMID: 31507608 Free PMC article.
References
-
- Allen TM, O’Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, et al. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407: 386–390 - PubMed
-
- Alter G, Teigen N, Ahern R, Streeck H, Meier A, Rosenberg ES, Altfeld M 2007b. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis 195: 1452–1460 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
