The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration
- PMID: 22761994
- PMCID: PMC3388469
- DOI: 10.1038/srep00488
The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration
Abstract
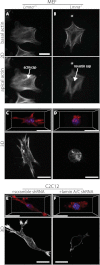
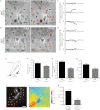
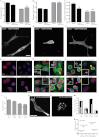
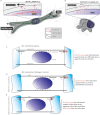
Cells often migrate in vivo in an extracellular matrix that is intrinsically three-dimensional (3D) and the role of actin filament architecture in 3D cell migration is less well understood. Here we show that, while recently identified linkers of nucleoskeleton to cytoskeleton (LINC) complexes play a minimal role in conventional 2D migration, they play a critical role in regulating the organization of a subset of actin filament bundles - the perinuclear actin cap - connected to the nucleus through Nesprin2giant and Nesprin3 in cells in 3D collagen I matrix. Actin cap fibers prolong the nucleus and mediate the formation of pseudopodial protrusions, which drive matrix traction and 3D cell migration. Disruption of LINC complexes disorganizes the actin cap, which impairs 3D cell migration. A simple mechanical model explains why LINC complexes and the perinuclear actin cap are essential in 3D migration by providing mechanical support to the formation of pseudopodial protrusions.
Figures

Similar articles
-
The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction.Sci Rep. 2013;3:1087. doi: 10.1038/srep01087. Epub 2013 Jan 18. Sci Rep. 2013. PMID: 23336069 Free PMC article.
-
The perinuclear actin cap in health and disease.Nucleus. 2010 Jul-Aug;1(4):337-42. doi: 10.4161/nucl.1.4.12331. Epub 2010 Apr 23. Nucleus. 2010. PMID: 21327082 Free PMC article.
-
Nuclear lamin isoforms differentially contribute to LINC complex-dependent nucleocytoskeletal coupling and whole-cell mechanics.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2121816119. doi: 10.1073/pnas.2121816119. Epub 2022 Apr 19. Proc Natl Acad Sci U S A. 2022. PMID: 35439057 Free PMC article.
-
Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function.Biochem Soc Trans. 2011 Dec;39(6):1729-34. doi: 10.1042/BST20110686. Biochem Soc Trans. 2011. PMID: 22103516 Free PMC article. Review.
-
The assembly and function of perinuclear actin cap in migrating cells.Protoplasma. 2017 May;254(3):1207-1218. doi: 10.1007/s00709-017-1077-0. Epub 2017 Jan 18. Protoplasma. 2017. PMID: 28101692 Review.
Cited by
-
Patient-Derived Glioma Models: From Patients to Dish to Animals.Cells. 2019 Sep 30;8(10):1177. doi: 10.3390/cells8101177. Cells. 2019. PMID: 31574953 Free PMC article. Review.
-
PCBP1 regulates the transcription and alternative splicing of metastasis‑related genes and pathways in hepatocellular carcinoma.Sci Rep. 2021 Dec 2;11(1):23356. doi: 10.1038/s41598-021-02642-z. Sci Rep. 2021. PMID: 34857818 Free PMC article.
-
Introduction to cell-hydrogel mechanosensing.Interface Focus. 2014 Apr 6;4(2):20130038. doi: 10.1098/rsfs.2013.0038. Interface Focus. 2014. PMID: 24748951 Free PMC article. Review.
-
The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction.Sci Rep. 2013;3:1087. doi: 10.1038/srep01087. Epub 2013 Jan 18. Sci Rep. 2013. PMID: 23336069 Free PMC article.
-
The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle.Cells. 2019 Jul 10;8(7):701. doi: 10.3390/cells8070701. Cells. 2019. PMID: 31295970 Free PMC article. Review.
References
-
- Pollard T. D. & Borisy G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003). - PubMed
-
- Ridley A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003). - PubMed
-
- Sahai E. & Marshall C. J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5, 711–719 (2003). - PubMed
-
- Kedrin D., van Rheenen J., Hernandez L., Condeelis J. & Segall J. E. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia 12, 143–152 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

