Amplitude of the SCN clock enhanced by the behavioral activity rhythm
- PMID: 22761873
- PMCID: PMC3386260
- DOI: 10.1371/journal.pone.0039693
Amplitude of the SCN clock enhanced by the behavioral activity rhythm
Abstract
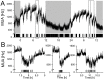
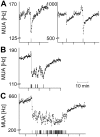
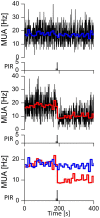
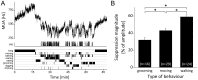
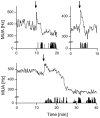
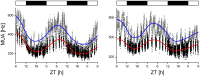
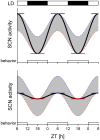
Circadian rhythms are regulated by the suprachiasmatic nucleus (SCN), a small structure at the base of the hypothalamus. While light effects on the SCN are well established, little is known of behavioral effects. This study elucidates direct modulating action of behavioral activity on the SCN by use of in vivo electrophysiology recordings, assessments of general locomotor behavior, and video-tracking of mice. The results show suppression of SCN neuronal activity by spontaneous behavior, the magnitude being dependent on the intensity, duration and type of behavioral activity. The suppression was moderate (32% of circadian amplitude) for low-intensity behavior and considerable (59%) for locomotor activity. Mild manipulation of the animals had reversed effects on the SCN indicating that different mechanisms are involved in the regulatory effect of spontaneous versus induced activity. The results indicate that exercise at the proper time of the cycle can boost the amplitude of the rhythm of the SCN clock itself. This has potentially beneficial effects for other rhythmic functions that are under the control of the SCN.
Conflict of interest statement
Figures
Similar articles
-
In vivo monitoring of multi-unit neural activity in the suprachiasmatic nucleus reveals robust circadian rhythms in Period1⁻/⁻ mice.PLoS One. 2013 May 22;8(5):e64333. doi: 10.1371/journal.pone.0064333. Print 2013. PLoS One. 2013. PMID: 23717599 Free PMC article.
-
Pet-1 deficiency alters the circadian clock and its temporal organization of behavior.PLoS One. 2014 May 15;9(5):e97412. doi: 10.1371/journal.pone.0097412. eCollection 2014. PLoS One. 2014. PMID: 24831114 Free PMC article.
-
Connexin30 and Connexin43 show a time-of-day dependent expression in the mouse suprachiasmatic nucleus and modulate rhythmic locomotor activity in the context of chronodisruption.Cell Commun Signal. 2019 Jun 11;17(1):61. doi: 10.1186/s12964-019-0370-2. Cell Commun Signal. 2019. PMID: 31186021 Free PMC article.
-
BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus.PLoS One. 2008;3(12):e3884. doi: 10.1371/journal.pone.0003884. Epub 2008 Dec 8. PLoS One. 2008. PMID: 19060951 Free PMC article.
-
[Mechanisms of structural plasticity associated with photic synchronization of the circadian clock within the suprachiasmatic nucleus].J Soc Biol. 2009;203(1):49-63. doi: 10.1051/jbio:2009004. Epub 2009 Apr 10. J Soc Biol. 2009. PMID: 19358811 Review. French.
Cited by
-
The cholinergic forebrain arousal system acts directly on the circadian pacemaker.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):13498-13503. doi: 10.1073/pnas.1610342113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821764 Free PMC article.
-
Time-shifting effects of methylphenidate on daily rhythms in the diurnal rodent Arvicanthis ansorgei.Psychopharmacology (Berl). 2018 Aug;235(8):2323-2333. doi: 10.1007/s00213-018-4928-2. Epub 2018 May 18. Psychopharmacology (Berl). 2018. PMID: 29777288
-
Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals.PLoS One. 2013;8(3):e56519. doi: 10.1371/journal.pone.0056519. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483886 Free PMC article. Clinical Trial.
-
Molecular mechanism of circadian rhythmicity of seizures in temporal lobe epilepsy.Front Cell Neurosci. 2012 Nov 23;6:55. doi: 10.3389/fncel.2012.00055. eCollection 2012. Front Cell Neurosci. 2012. PMID: 23189039 Free PMC article.
-
Interacting epidemics? Sleep curtailment, insulin resistance, and obesity.Ann N Y Acad Sci. 2012 Aug;1264(1):110-34. doi: 10.1111/j.1749-6632.2012.06655.x. Epub 2012 Jul 24. Ann N Y Acad Sci. 2012. PMID: 22827862 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

