Ginger stimulates hematopoiesis via Bmp pathway in zebrafish
- PMID: 22761764
- PMCID: PMC3382625
- DOI: 10.1371/journal.pone.0039327
Ginger stimulates hematopoiesis via Bmp pathway in zebrafish
Abstract
Background: Anemia is a hematologic disorder with decreased number of erythrocytes. Erythropoiesis, the process by which red blood cells differentiate, are conserved in humans, mice and zebrafish. The only known agents available to treat pathological anemia are erythropoietin and its biologic derivatives. However, erythropoietin therapy elicits unwanted side-effects, high cost and intravenous or subcutaneous injection, warranting the development of a more cost effective and non-peptide alternative. Ginger (Zingiber officinale) has been widely used in traditional medicine; however, to date there is no scientific research documenting the potential of ginger to stimulate hematopoiesis.
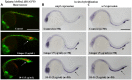
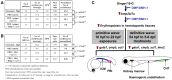
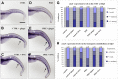
Methodology/principal findings: Here, we utilized gata1:dsRed transgenic zebrafish embryos to investigate the effect of ginger extract on hematopoiesis in vivo and we identified its bioactive component, 10-gingerol. We confirmed that ginger and 10-gingerol promote the expression of gata1 in erythroid cells and increase the expression of hematopoietic progenitor markers cmyb and scl. We also demonstrated that ginger and 10-gingerol can promote the hematopoietic recovery from acute hemolytic anemia in zebrafish, by quantifying the number of circulating erythroid cells in the dorsal aorta using video microscopy. We found that ginger and 10-gingerol treatment during gastrulation results in an increase of bmp2b and bmp7a expression, and their downstream effectors, gata2 and eve1. At later stages ginger and 10-gingerol can induce bmp2b/7a, cmyb, scl and lmo2 expression in the caudal hematopoietic tissue area. We further confirmed that Bmp/Smad pathway mediates this hematopoiesis promoting effect of ginger by using the Bmp-activated Bmp type I receptor kinase inhibitors dorsomorphin, LND193189 and DMH1.
Conclusions/significance: Our study provides a strong foundation to further evaluate the molecular mechanism of ginger and its bioactive components during hematopoiesis and to investigate their effects in adults. Our results will provide the basis for future research into the effect of ginger during mammalian hematopoiesis to develop novel erythropoiesis promoting agents.
Conflict of interest statement
Figures




Similar articles
-
Rescue of hematopoietic stem/progenitor cells formation in plcg1 zebrafish mutant.Sci Rep. 2019 Jan 21;9(1):244. doi: 10.1038/s41598-018-36338-8. Sci Rep. 2019. PMID: 30664660 Free PMC article.
-
Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1ɑ Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol.J Food Sci. 2019 Aug;84(8):2101-2111. doi: 10.1111/1750-3841.14723. Epub 2019 Aug 1. J Food Sci. 2019. PMID: 31369153
-
Regulation of hematopoiesis by the BMP signaling pathway in adult zebrafish.Exp Hematol. 2008 Dec;36(12):1604-1615. doi: 10.1016/j.exphem.2008.08.005. Epub 2008 Oct 29. Exp Hematol. 2008. PMID: 18973974 Free PMC article.
-
Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review.Phytother Res. 2018 Oct;32(10):1885-1907. doi: 10.1002/ptr.6134. Epub 2018 Jul 16. Phytother Res. 2018. PMID: 30009484 Review.
-
Ginger: A Novel Strategy to Battle Cancer through Modulating Cell Signalling Pathways: A Review.Curr Pharm Biotechnol. 2019;20(1):5-16. doi: 10.2174/1389201020666190119142331. Curr Pharm Biotechnol. 2019. PMID: 30659535 Review.
Cited by
-
Using zebrafish to model erythroid lineage toxicity and regeneration.Haematologica. 2016 May;101(5):e164-7. doi: 10.3324/haematol.2016.142562. Epub 2016 Mar 4. Haematologica. 2016. PMID: 26944471 Free PMC article. No abstract available.
-
A versatile, automated and high-throughput drug screening platform for zebrafish embryos.Biol Open. 2021 Sep 15;10(9):bio058513. doi: 10.1242/bio.058513. Epub 2021 Sep 2. Biol Open. 2021. PMID: 34472582 Free PMC article.
-
Is 6-Shogaol an Effective Phytochemical for Patients With Lower-risk Myelodysplastic Syndrome? A Narrative Review.Integr Cancer Ther. 2021 Jan-Dec;20:15347354211065038. doi: 10.1177/15347354211065038. Integr Cancer Ther. 2021. PMID: 34930049 Free PMC article. Review.
-
Todralazine protects zebrafish from lethal effects of ionizing radiation: role of hematopoietic cell expansion.Zebrafish. 2015 Feb;12(1):33-47. doi: 10.1089/zeb.2014.0992. Epub 2014 Dec 17. Zebrafish. 2015. PMID: 25517940 Free PMC article.
-
Targeted therapy of human leukemia xenografts in immunodeficient zebrafish.Sci Rep. 2021 Mar 11;11(1):5715. doi: 10.1038/s41598-021-85141-5. Sci Rep. 2021. PMID: 33707624 Free PMC article.
References
-
- Snyder A, Fraser ST, Baron MH. Bone morphogenetic proteins in vertebrate hematopoietic development. J Cell Biochem. 2004;93:224–232. - PubMed
-
- Grassinger J, Simon M, Mueller G, Drewel D, Andreesen R, et al. Bone morphogenetic protein (BMP)-7 but not BMP-2 and BMP-4 improves maintenance of primitive peripheral blood-derived hematopoietic progenitor cells (HPC) cultured in serum-free medium supplemented with early acting cytokines. Cytokine. 2007;40:165–171. - PubMed
-
- Schmerer M, Evans T. Primitive erythropoiesis is regulated by Smad-dependent signaling in postgastrulation mesoderm. Blood. 2003;102:3196–3205. - PubMed
-
- Burns CE, DeBlasio T, Zhou Y, Zhang J, Zon L, et al. Isolation and characterization of runxa and runxb, zebrafish members of the runt family of transcriptional regulators. Exp Hematol. 2002;30:1381–1389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

