Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination
- PMID: 22761693
- PMCID: PMC3380899
- DOI: 10.1371/journal.pone.0038658
Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination
Abstract
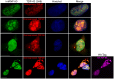
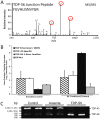
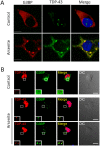
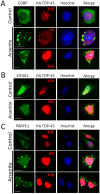
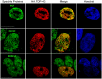
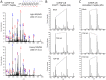
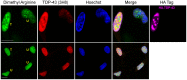
TAR DNA-binding protein 43 (TDP-43) is a major component within ubiquitin-positive inclusions of a number of neurodegenerative diseases that increasingly are considered as TDP-43 proteinopathies. Identities of other inclusion proteins associated with TDP-43 aggregation remain poorly defined. In this study, we identify and quantitate 35 co-aggregating proteins in the detergent-resistant fraction of HEK-293 cells in which TDP-43 or a particularly aggregate prone variant, TDP-S6, were enriched following overexpression, using stable isotope-labeled (SILAC) internal standards and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). We also searched for differential post-translational modification (PTM) sites of ubiquitination. Four sites of ubiquitin conjugation to TDP-43 or TDP-S6 were confirmed by dialkylated GST-TDP-43 external reference peptides, occurring on or near RNA binding motif (RRM) 1. RRM-containing proteins co-enriched in cytoplasmic granular structures in HEK-293 cells and primary motor neurons with insoluble TDP-S6, including cytoplasmic stress granule associated proteins G3BP, PABPC1, and eIF4A1. Proteomic evidence for TDP-43 co-aggregation with paraspeckle markers RBM14, PSF and NonO was also validated by western blot and by immunocytochemistry in HEK-293 cells. An increase in peptides from methylated arginine-glycine-glycine (RGG) RNA-binding motifs of FUS/TLS and hnRNPs was found in the detergent-insoluble fraction of TDP-overexpressing cells. Finally, TDP-43 and TDP-S6 detergent-insoluble species were reduced by mutagenesis of the identified ubiquitination sites, even following oxidative or proteolytic stress. Together, these findings define some of the aggregation partners of TDP-43, and suggest that TDP-43 ubiquitination influences TDP-43 oligomerization.
Conflict of interest statement
Figures



Similar articles
-
Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains.Mol Cell Proteomics. 2010 Apr;9(4):705-18. doi: 10.1074/mcp.M800390-MCP200. Epub 2010 Jan 4. Mol Cell Proteomics. 2010. PMID: 20047951 Free PMC article.
-
Quantitative analysis of the detergent-insoluble brain proteome in frontotemporal lobar degeneration using SILAC internal standards.J Proteome Res. 2012 May 4;11(5):2721-38. doi: 10.1021/pr2010814. Epub 2012 Apr 4. J Proteome Res. 2012. PMID: 22416763 Free PMC article.
-
RNP2 of RNA recognition motif 1 plays a central role in the aberrant modification of TDP-43.PLoS One. 2013 Jun 28;8(6):e66966. doi: 10.1371/journal.pone.0066966. Print 2013. PLoS One. 2013. PMID: 23840565 Free PMC article.
-
TDP-43: a DNA and RNA binding protein with roles in neurodegenerative diseases.Int J Biochem Cell Biol. 2010 Oct;42(10):1606-9. doi: 10.1016/j.biocel.2010.06.016. Epub 2010 Jun 25. Int J Biochem Cell Biol. 2010. PMID: 20601083 Review.
-
TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration.Mol Cell Neurosci. 2019 Oct;100:103396. doi: 10.1016/j.mcn.2019.103396. Epub 2019 Aug 21. Mol Cell Neurosci. 2019. PMID: 31445085 Free PMC article. Review.
Cited by
-
TDP43 autoregulation gives rise to shortened isoforms that are tightly controlled by both transcriptional and post-translational mechanisms.bioRxiv [Preprint]. 2024 Jul 4:2024.07.02.601776. doi: 10.1101/2024.07.02.601776. bioRxiv. 2024. Update in: Cell Rep. 2025 Jan 28;44(1):115113. doi: 10.1016/j.celrep.2024.115113 PMID: 39005384 Free PMC article. Updated. Preprint.
-
Calcium-responsive transactivator (CREST) protein shares a set of structural and functional traits with other proteins associated with amyotrophic lateral sclerosis.Mol Neurodegener. 2015 Apr 10;10:20. doi: 10.1186/s13024-015-0014-y. Mol Neurodegener. 2015. PMID: 25888396 Free PMC article.
-
Identification and characterization of ubiquitinylation sites in TAR DNA-binding protein of 43 kDa (TDP-43).J Biol Chem. 2018 Oct 12;293(41):16083-16099. doi: 10.1074/jbc.RA118.003440. Epub 2018 Aug 17. J Biol Chem. 2018. PMID: 30120199 Free PMC article.
-
Abnormal gephyrin immunoreactivity associated with Alzheimer disease pathologic changes.J Neuropathol Exp Neurol. 2013 Nov;72(11):1009-15. doi: 10.1097/01.jnen.0000435847.59828.db. J Neuropathol Exp Neurol. 2013. PMID: 24128675 Free PMC article.
-
Quantitative Analysis of the Brain Ubiquitylome in Alzheimer's Disease.Proteomics. 2018 Oct;18(20):e1800108. doi: 10.1002/pmic.201800108. Proteomics. 2018. PMID: 30230243 Free PMC article.
References
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
-
- Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. - PubMed
-
- Ayala YM, Zago P, D’Ambrogio A, Xu YF, Petrucelli L, et al. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. - PubMed
-
- Mackenzie IR, Rademakers R. The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenetics. 2007. - PubMed
-
- Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

