A tethering complex dimer catalyzes trans-SNARE complex formation in intracellular membrane fusion
- PMID: 22754631
- PMCID: PMC3383723
- DOI: 10.4161/bioa.20359
A tethering complex dimer catalyzes trans-SNARE complex formation in intracellular membrane fusion
Abstract
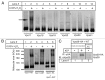
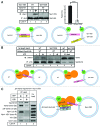
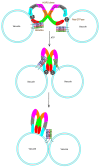
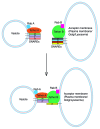
SNARE complexes mediate membrane fusion in the endomembrane system. They consist of coiled-coil bundles of four helices designated as Qa, Qb, Qc and R. A critical intermediate in the fusion pathway is the trans-SNARE complex generated by the assembly of SNAREs residing in opposing membranes. Mechanistic details of trans-SNARE complex formation and topology in a physiological system remain largely unresolved. Our studies on native yeast vacuoles revealed that SNAREs alone are insufficient to form trans-SNARE complexes and that additional factors, potentially tethering complexes and Rab GTPases, are required for the process. Here we report a novel finding that a HOPS tethering complex dimer catalyzes Rab GTPase-dependent formation of a topologically preferred QbQcR-Qa trans-SNARE complex.
Figures

Similar articles
-
Assembly of intermediates for rapid membrane fusion.J Biol Chem. 2018 Jan 26;293(4):1346-1352. doi: 10.1074/jbc.RA117.000791. Epub 2017 Dec 5. J Biol Chem. 2018. PMID: 29208657 Free PMC article.
-
Sequential analysis of trans-SNARE formation in intracellular membrane fusion.PLoS Biol. 2012 Jan;10(1):e1001243. doi: 10.1371/journal.pbio.1001243. Epub 2012 Jan 17. PLoS Biol. 2012. PMID: 22272185 Free PMC article.
-
PI3P regulates multiple stages of membrane fusion.Mol Biol Cell. 2023 Mar 1;34(3):ar17. doi: 10.1091/mbc.E22-10-0486. Epub 2023 Feb 3. Mol Biol Cell. 2023. PMID: 36735517 Free PMC article.
-
Membrane dynamics and fusion at late endosomes and vacuoles--Rab regulation, multisubunit tethering complexes and SNAREs.Eur J Cell Biol. 2011 Sep;90(9):779-85. doi: 10.1016/j.ejcb.2011.04.007. Epub 2011 Jun 16. Eur J Cell Biol. 2011. PMID: 21683469 Review.
-
Functions of SNAREs in intracellular membrane fusion and lipid bilayer mixing.J Cell Sci. 2005 Sep 1;118(Pt 17):3819-28. doi: 10.1242/jcs.02561. J Cell Sci. 2005. PMID: 16129880 Review.
Cited by
-
The Vesicle Priming Factor CAPS Functions as a Homodimer via C2 Domain Interactions to Promote Regulated Vesicle Exocytosis.J Biol Chem. 2016 Sep 30;291(40):21257-21270. doi: 10.1074/jbc.M116.728097. Epub 2016 Aug 15. J Biol Chem. 2016. PMID: 27528604 Free PMC article.
-
Dynamin-SNARE interactions control trans-SNARE formation in intracellular membrane fusion.Nat Commun. 2013;4:1704. doi: 10.1038/ncomms2724. Nat Commun. 2013. PMID: 23591871 Free PMC article.
-
A dynamin homolog promotes the transition from hemifusion to content mixing in intracellular membrane fusion.Traffic. 2014 May;15(5):558-71. doi: 10.1111/tra.12156. Epub 2014 Feb 25. Traffic. 2014. PMID: 24471450 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
