Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM
- PMID: 22753497
- PMCID: PMC3406826
- DOI: 10.1073/pnas.1204935109
Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM
Abstract
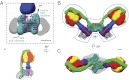
Mitochondrial ATP synthase is responsible for the synthesis of ATP, a universal energy currency in cells. Whereas X-ray crystallography has revealed the structure of the soluble region of the complex and the membrane-intrinsic c-subunits, little is known about the structure of the six other proteins (a, b, f, A6L, e, and g) that comprise the membrane-bound region of the complex in animal mitochondria. Here, we present the structure of intact bovine mitochondrial ATP synthase at ∼18 Å resolution by electron cryomicroscopy of single particles in amorphous ice. The map reveals that the a-subunit and c(8)-ring of the complex interact with a small contact area and that the b-subunit spans the membrane without contacting the c(8)-ring. The e- and g-subunits extend from the a-subunit density distal to the c(8)-ring. The map was calculated from images of a preparation of the enzyme solubilized with the detergent dodecyl maltoside, which is visible in electron cryomicroscopy maps. The structure shows that the micelle surrounding the complex is curved. The observed bend in the micelle of the detergent-solubilized complex is consistent with previous electron tomography experiments and suggests that monomers of ATP synthase are sufficient to produce curvature in lipid bilayers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

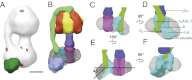
Similar articles
-
Cryo-EM structure of the yeast ATP synthase.J Mol Biol. 2008 Oct 24;382(5):1256-64. doi: 10.1016/j.jmb.2008.08.014. Epub 2008 Aug 12. J Mol Biol. 2008. PMID: 18722382
-
Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM.Elife. 2015 Oct 6;4:e10180. doi: 10.7554/eLife.10180. Elife. 2015. PMID: 26439008 Free PMC article.
-
Cryo-EM of ATP synthases.Curr Opin Struct Biol. 2018 Oct;52:71-79. doi: 10.1016/j.sbi.2018.08.005. Epub 2018 Sep 18. Curr Opin Struct Biol. 2018. PMID: 30240940 Review.
-
Structure of a Complete ATP Synthase Dimer Reveals the Molecular Basis of Inner Mitochondrial Membrane Morphology.Mol Cell. 2016 Aug 4;63(3):445-56. doi: 10.1016/j.molcel.2016.05.037. Epub 2016 Jun 30. Mol Cell. 2016. PMID: 27373333 Free PMC article.
-
Structure and Mechanisms of F-Type ATP Synthases.Annu Rev Biochem. 2019 Jun 20;88:515-549. doi: 10.1146/annurev-biochem-013118-110903. Epub 2019 Mar 22. Annu Rev Biochem. 2019. PMID: 30901262 Review.
Cited by
-
Understanding structure, function, and mutations in the mitochondrial ATP synthase.Microb Cell. 2015 Apr 1;2(4):105-125. doi: 10.15698/mic2015.04.197. Microb Cell. 2015. PMID: 25938092 Free PMC article.
-
Efficient implementation of constant pH molecular dynamics on modern graphics processors.J Comput Chem. 2016 Sep 15;37(24):2171-80. doi: 10.1002/jcc.24435. Epub 2016 Jul 12. J Comput Chem. 2016. PMID: 27405884 Free PMC article.
-
Bovine F1Fo ATP synthase monomers bend the lipid bilayer in 2D membrane crystals.Elife. 2015 Mar 27;4:e06119. doi: 10.7554/eLife.06119. Elife. 2015. PMID: 25815585 Free PMC article.
-
The cristae modulator Optic atrophy 1 requires mitochondrial ATP synthase oligomers to safeguard mitochondrial function.Nat Commun. 2018 Aug 24;9(1):3399. doi: 10.1038/s41467-018-05655-x. Nat Commun. 2018. PMID: 30143614 Free PMC article.
-
The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: emerging new roles in mitochondrial pathophysiology.Int J Mol Sci. 2014 Apr 30;15(5):7513-36. doi: 10.3390/ijms15057513. Int J Mol Sci. 2014. PMID: 24786291 Free PMC article.
References
-
- Dunn SD, Heppel LA. Properties and functions of the subunits of the Escherichia coli coupling factor ATPase. Arch Biochem Biophys. 1981;210:421–436. - PubMed
-
- Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. - PubMed
-
- Collinson IR, et al. Fo membrane domain of ATP synthase from bovine heart mitochondria: Purification, subunit composition, and reconstitution with F1-ATPase. Biochemistry. 1994;33:7971–7978. - PubMed
-
- Collinson IR, et al. ATP synthase from bovine heart mitochondria. In vitro assembly of a stalk complex in the presence of F1-ATPase and in its absence. J Mol Biol. 1994;242:408–421. - PubMed
-
- Collinson IR, Skehel JM, Fearnley IM, Runswick MJ, Walker JE. The F1F0-ATPase complex from bovine heart mitochondria: The molar ratio of the subunits in the stalk region linking the F1 and F0 domains. Biochemistry. 1996;35:12640–12646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

