Verapamil- and state-dependent effect of 2-aminoethylmethanethiosulphonate (MTSEA) on hK(v)1.3 channels
- PMID: 22748056
- PMCID: PMC3505002
- DOI: 10.1111/j.1476-5381.2012.02092.x
Verapamil- and state-dependent effect of 2-aminoethylmethanethiosulphonate (MTSEA) on hK(v)1.3 channels
Abstract
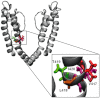
Background and purpose: T-cells usually express voltage-gated K(v) 1.3 channels. These channels are distinguished by their typical C-type inactivation. Therefore, to be able to rationally design drugs specific for the C-type inactivation state that may have therapeutic value in autoimmune disease therapy, it is necessary to identify those amino acids that are accessible for drug binding in C-type inactivated channels.
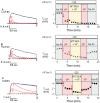
Experimental approach: The influence of 2-aminoethylmethanethiosulphonate (MTSEA) on currents through wild-type human K(v)1.3 (hK(v)1.3) and three mutant channels, hK(v)1.3_L418C, hK(v)1.3_T419C and hK(v)1.3_I420C, in the closed, open and inactivated states was investigated by the patch-clamp technique.
Key results: Currents through hK(v)1.3_L418C and hK(v)1.3_T419C channels were irreversibly reduced after the external application of MTSEA in the open state but not in the inactivated and closed states. Currents through hK(v)1.3_I420C channels were irreversibly reduced in the open and inactivated states but not in the closed state. In the presence of verapamil, the MTSEA modification of hK(v)1.3_T419C and hK(v)1.3_I420C channels was prevented, while the MTSEA modification of hK(v)1.3_L418C channels was unaffected.
Conclusion and implications: From our experiments, we conclude that the activation gate of all mutant channels must be open for modification by MTSEA and must also be open during inactivation. In addition, the relative movement of the S6 segments that occur during C-type inactivation includes a movement of the side chains of the amino acids at positions 418 and 419 away from the pore lining. Furthermore, the overlapping binding site for MTSEA and verapamil does not include position 418 in hK(v) 1.3 channels.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures


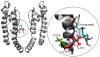
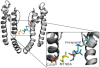
Similar articles
-
Effect of verapamil on the action of methanethiosulfonate reagents on human voltage-gated K(v)1.3 channels: implications for the C-type inactivated state.Br J Pharmacol. 2011 Jun;163(3):662-74. doi: 10.1111/j.1476-5381.2011.01258.x. Br J Pharmacol. 2011. PMID: 21306584 Free PMC article.
-
Kinetic Aspects of Verapamil Binding (On-Rate) on Wild-Type and Six hKv1.3 Mutant Channels.Cell Physiol Biochem. 2017;44(1):172-184. doi: 10.1159/000484625. Epub 2017 Nov 6. Cell Physiol Biochem. 2017. PMID: 29131061
-
Effect of K+ and Rb+ on the action of verapamil on a voltage-gated K+ channel, hKv1.3: implications for a second open state?Br J Pharmacol. 2009 Jul;157(5):757-68. doi: 10.1111/j.1476-5381.2009.00202.x. Epub 2009 Apr 9. Br J Pharmacol. 2009. PMID: 19371328 Free PMC article.
-
Evidence for a deep pore activation gate in small conductance Ca2+-activated K+ channels.J Gen Physiol. 2007 Dec;130(6):601-10. doi: 10.1085/jgp.200709828. Epub 2007 Nov 12. J Gen Physiol. 2007. PMID: 17998394 Free PMC article.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
Cited by
-
Loureirin B, an essential component of Sanguis Draxonis, inhibits Kv1.3 channel and suppresses cytokine release from Jurkat T cells.Cell Biosci. 2014 Dec 12;4:78. doi: 10.1186/2045-3701-4-78. eCollection 2014. Cell Biosci. 2014. PMID: 25937895 Free PMC article.
References
-
- Cordero-Morales JF, Cuello LG, Zhao Y, Jogini V, Cortes DM, Roux B, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

