Dietary soyasaponin supplementation to pea protein concentrate reveals nutrigenomic interactions underlying enteropathy in Atlantic salmon (Salmo salar)
- PMID: 22748053
- PMCID: PMC3424111
- DOI: 10.1186/1746-6148-8-101
Dietary soyasaponin supplementation to pea protein concentrate reveals nutrigenomic interactions underlying enteropathy in Atlantic salmon (Salmo salar)
Abstract
Background: Use of plant ingredients in aquaculture feeds is impeded by high contents of antinutritional factors such as saponins, which may cause various pharmacological and biological effects. In this study, transcriptome changes were analyzed using a 21 k oligonucleotide microarray and qPCR in the distal intestine of Atlantic salmon fed diets based on five plant protein sources combined with soybean saponins.
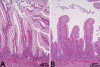
Results: Diets with corn gluten, sunflower, rapeseed or horsebean produced minor effects while the combination of saponins with pea protein concentrate caused enteritis and major transcriptome changes. Acute inflammation was characterised by up-regulation of cytokines, NFkB and TNFalpha related genes and regulators of T-cell function, while the IFN-axis was suppressed. Induction of lectins, complement, metalloproteinases and the respiratory burst complex parallelled a down-regulation of genes for free radical scavengers and iron binding proteins. Marked down-regulation of xenobiotic metabolism was also observed, possibly increasing vulnerability of the intestinal tissue. A hallmark of metabolic changes was dramatic down-regulation of lipid, bile and steroid metabolism. Impairment of digestion was further suggested by expression changes of nutrient transporters and regulators of water balance (e.g. aquaporin, guanylin). On the other hand, microarray profiling revealed activation of multiple mucosal defence processes. Annexin-1, with important anti-inflammatory and gastroprotective properties, was markedly up-regulated. Furthermore, augmented synthesis of polyamines needed for cellular proliferation (up-regulation of arginase and ornithine decarboxylase) and increased mucus production (down-regulation of glycan turnover and goblet cell hyperplasia) could participate in mucosal healing and restoration of normal tissue function.
Conclusion: The current study promoted understanding of salmon intestinal pathology and establishment of a model for feed induced enteritis. Multiple gene expression profiling further characterised the inflammation and described the intestinal pathology at the molecular level.
Figures

Similar articles
-
Interaction of soyasaponins with plant ingredients in diets for Atlantic salmon, Salmo salar L.Br J Nutr. 2012 Jun;107(11):1570-90. doi: 10.1017/S0007114511004892. Epub 2011 Sep 14. Br J Nutr. 2012. PMID: 21914238 Clinical Trial.
-
Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis.Fish Shellfish Immunol. 2013 Feb;34(2):599-609. doi: 10.1016/j.fsi.2012.11.031. Epub 2012 Dec 11. Fish Shellfish Immunol. 2013. PMID: 23246810
-
Differential responses of the gut transcriptome to plant protein diets in farmed Atlantic salmon.BMC Genomics. 2016 Feb 29;17:156. doi: 10.1186/s12864-016-2473-0. BMC Genomics. 2016. PMID: 26925977 Free PMC article.
-
Changes in the liver transcriptome of farmed Atlantic salmon (Salmo salar) fed experimental diets based on terrestrial alternatives to fish meal and fish oil.BMC Genomics. 2018 Nov 3;19(1):796. doi: 10.1186/s12864-018-5188-6. BMC Genomics. 2018. PMID: 30390635 Free PMC article.
-
Transcriptomic responses to functional feeds in Atlantic salmon (Salmo salar).Fish Shellfish Immunol. 2011 Nov;31(5):704-15. doi: 10.1016/j.fsi.2011.02.023. Epub 2011 Mar 4. Fish Shellfish Immunol. 2011. PMID: 21377530 Review.
Cited by
-
Tryptophan metabolism and gut flora profile in different soybean protein induced enteritis of pearl gentian groupers.Front Nutr. 2022 Dec 19;9:1014502. doi: 10.3389/fnut.2022.1014502. eCollection 2022. Front Nutr. 2022. PMID: 36601073 Free PMC article.
-
Soybean Meal-Dependent Intestinal Inflammation Induces Different Patterns of Bone-Loss in Adult Zebrafish Scale.Biomedicines. 2021 Apr 6;9(4):393. doi: 10.3390/biomedicines9040393. Biomedicines. 2021. PMID: 33917641 Free PMC article.
-
Ontogeny of the Digestive System of Atlantic Salmon (Salmo salar L.) and Effects of Soybean Meal from Start-Feeding.PLoS One. 2015 Apr 29;10(4):e0124179. doi: 10.1371/journal.pone.0124179. eCollection 2015. PLoS One. 2015. PMID: 25923375 Free PMC article.
-
Effect of yeast species and processing on intestinal microbiota of Atlantic salmon (Salmo salar) fed soybean meal-based diets in seawater.Anim Microbiome. 2023 Apr 4;5(1):21. doi: 10.1186/s42523-023-00242-y. Anim Microbiome. 2023. PMID: 37016467 Free PMC article.
-
Impact of down-stream processing on functional properties of yeasts and the implications on gut health of Atlantic salmon (Salmo salar).Sci Rep. 2021 Feb 24;11(1):4496. doi: 10.1038/s41598-021-83764-2. Sci Rep. 2021. PMID: 33627754 Free PMC article.
References
-
- Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu GS, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquaculture Res. 2007;38:551–579. doi: 10.1111/j.1365-2109.2007.01704.x. - DOI
-
- Francis G, Makkar HPS, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199:197–227. doi: 10.1016/S0044-8486(01)00526-9. - DOI
-
- Krogdahl Å, Penn M, Thorsen J, Refstie S, Bakke AM. Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquaculture Res. 2010;41:333–344. doi: 10.1111/j.1365-2109.2009.02426.x. - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

