SUMOylation represses Nanog expression via modulating transcription factors Oct4 and Sox2
- PMID: 22745796
- PMCID: PMC3382131
- DOI: 10.1371/journal.pone.0039606
SUMOylation represses Nanog expression via modulating transcription factors Oct4 and Sox2
Abstract
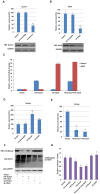
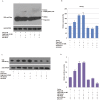
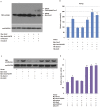
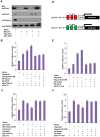
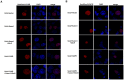
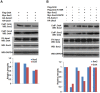
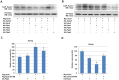
Nanog is a pivotal transcription factor in embryonic stem (ES) cells and is essential for maintaining the pluripotency and self-renewal of ES cells. SUMOylation has been proved to regulate several stem cell markers' function, such as Oct4 and Sox2. Nanog is strictly regulated by Oct4/Sox2 heterodimer. However, the direct effects of SUMOylation on Nanog expression remain unclear. In this study, we reported that SUMOylation repressed Nanog expression. Depletion of Sumo1 or its conjugating enzyme Ubc9 increased the expression of Nanog, while high SUMOylation reduced its expression. Interestingly, we found that SUMOylation of Oct4 and Sox2 regulated Nanog in an opposing manner. SUMOylation of Oct4 enhanced Nanog expression, while SUMOylated Sox2 inhibited its expression. Moreover, SUMOylation of Oct4 by Pias2 or Sox2 by Pias3 impaired the interaction between Oct4 and Sox2. Taken together, these results indicate that SUMOylation has a negative effect on Nanog expression and provides new insights into the mechanism of SUMO modification involved in ES cells regulation.
Conflict of interest statement
Figures
Similar articles
-
Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma.J Biol Chem. 2012 Sep 21;287(39):32800-24. doi: 10.1074/jbc.M111.308528. Epub 2012 Jul 30. J Biol Chem. 2012. PMID: 22847005 Free PMC article.
-
Ethanol alters the balance of Sox2, Oct4, and Nanog expression in distinct subpopulations during differentiation of embryonic stem cells.Stem Cells Dev. 2013 Aug 1;22(15):2196-210. doi: 10.1089/scd.2012.0513. Epub 2013 Apr 9. Stem Cells Dev. 2013. PMID: 23470161 Free PMC article.
-
Expression of SOX2, NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions.Reprod Biol Endocrinol. 2017 Mar 3;15(1):14. doi: 10.1186/s12958-017-0234-9. Reprod Biol Endocrinol. 2017. PMID: 28253866 Free PMC article.
-
Networks of Transcription Factors for Oct4 Expression in Mice.DNA Cell Biol. 2017 Sep;36(9):725-736. doi: 10.1089/dna.2017.3780. Epub 2017 Jul 21. DNA Cell Biol. 2017. PMID: 28731785 Review.
-
Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis.Biochim Biophys Acta. 2016 Jun;1859(6):780-91. doi: 10.1016/j.bbagrm.2016.03.006. Epub 2016 Mar 23. Biochim Biophys Acta. 2016. PMID: 26992828 Review.
Cited by
-
SUMO-dependent transcriptional repression by Sox2 inhibits the proliferation of neural stem cells.PLoS One. 2024 Mar 20;19(3):e0298818. doi: 10.1371/journal.pone.0298818. eCollection 2024. PLoS One. 2024. PMID: 38507426 Free PMC article.
-
Temporal and SUMO-specific SUMOylation contribute to the dynamics of Polo-like kinase 1 (PLK1) and spindle integrity during mouse oocyte meiosis.Dev Biol. 2018 Feb 15;434(2):278-291. doi: 10.1016/j.ydbio.2017.12.011. Epub 2017 Dec 19. Dev Biol. 2018. PMID: 29269218 Free PMC article.
-
Embryonic Cells Redistribute SUMO1 upon Forced SUMO1 Overexpression.mBio. 2019 Dec 3;10(6):e01856-19. doi: 10.1128/mBio.01856-19. mBio. 2019. PMID: 31796536 Free PMC article.
-
Protein sumoylation in normal and cancer stem cells.Front Mol Biosci. 2022 Dec 19;9:1095142. doi: 10.3389/fmolb.2022.1095142. eCollection 2022. Front Mol Biosci. 2022. PMID: 36601585 Free PMC article. Review.
-
Cyclin-dependent kinase-mediated Sox2 phosphorylation enhances the ability of Sox2 to establish the pluripotent state.J Biol Chem. 2015 Sep 11;290(37):22782-94. doi: 10.1074/jbc.M115.658195. Epub 2015 Jul 2. J Biol Chem. 2015. PMID: 26139602 Free PMC article.
References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
-
- Lavial F, Acloque H, Bertocchini F, Macleod DJ, Boast S, et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development. 2007;134:3549–3563. - PubMed
-
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

