Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors
- PMID: 22745501
- PMCID: PMC3752062
- DOI: 10.1523/JNEUROSCI.0918-12.2012
Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors
Abstract
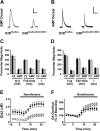
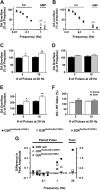
Dysfunctions of dopaminergic homeostasis leading to either low or high dopamine (DA) levels are causally linked to Parkinson's disease, schizophrenia, and addiction. Major sites of DA synthesis are the mesencephalic neurons originating in the substantia nigra and ventral tegmental area; these structures send major projections to the dorsal striatum (DSt) and nucleus accumbens (NAcc), respectively. DA finely tunes its own synthesis and release by activating DA D2 receptors (D2R). To date, this critical D2R-dependent function was thought to be solely due to activation of D2Rs on dopaminergic neurons (D2 autoreceptors); instead, using site-specific D2R knock-out mice, we uncover that D2 heteroreceptors located on non-DAergic medium spiny neurons participate in the control of DA levels. This D2 heteroreceptor-mediated mechanism is more efficient in the DSt than in NAcc, indicating that D2R signaling differentially regulates mesolimbic- versus nigrostriatal-mediated functions. This study reveals previously unappreciated control of DA signaling, shedding new light on region-specific regulation of DA-mediated effects.
Figures
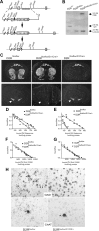
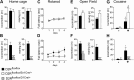
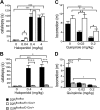
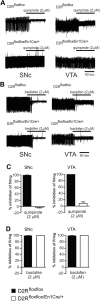
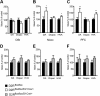

 , shell NAcc; F(1,27) = 4.34, p = 0.046). G, Comparison of the DA release probability in D2R-null (■), D2Rfloxflox/En1Cre/+ (□), and D2Rfloxflox/D1Cre/+ (▩) mice. Summary diagram of the difference between the D2R-null, D2Rfloxflox/En1Cre/+, and D2Rfloxflox/D1Cre/+ mice (as indicated) compared with their respective WT controls in the paired-pulse and train of pulses experiments. The frequencies represent interpulse intervals of 60, 30, 20, 10, 5, 1, 0.5, and 0.05 s. Two-way ANOVA with Bonferroni's post hoc test for genotype was used in all panels. *p < 0.05, **p < 0.01, ***p < 0.001.
, shell NAcc; F(1,27) = 4.34, p = 0.046). G, Comparison of the DA release probability in D2R-null (■), D2Rfloxflox/En1Cre/+ (□), and D2Rfloxflox/D1Cre/+ (▩) mice. Summary diagram of the difference between the D2R-null, D2Rfloxflox/En1Cre/+, and D2Rfloxflox/D1Cre/+ mice (as indicated) compared with their respective WT controls in the paired-pulse and train of pulses experiments. The frequencies represent interpulse intervals of 60, 30, 20, 10, 5, 1, 0.5, and 0.05 s. Two-way ANOVA with Bonferroni's post hoc test for genotype was used in all panels. *p < 0.05, **p < 0.01, ***p < 0.001.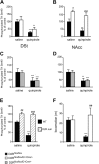
Similar articles
-
Dopamine D2 receptor desensitization by dopamine or corticotropin releasing factor in ventral tegmental area neurons is associated with increased glutamate release.Neuropharmacology. 2014 Jul;82:28-40. doi: 10.1016/j.neuropharm.2014.03.006. Epub 2014 Mar 19. Neuropharmacology. 2014. PMID: 24657149 Free PMC article.
-
Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors.Neuropharmacology. 2014 Jun;81:283-91. doi: 10.1016/j.neuropharm.2014.02.007. Epub 2014 Feb 22. Neuropharmacology. 2014. PMID: 24565640
-
Homeostatic regulation of excitatory synapses on striatal medium spiny neurons expressing the D2 dopamine receptor.Brain Struct Funct. 2016 May;221(4):2093-107. doi: 10.1007/s00429-015-1029-4. Epub 2015 Mar 18. Brain Struct Funct. 2016. PMID: 25782435
-
The role of D2-autoreceptors in regulating dopamine neuron activity and transmission.Neuroscience. 2014 Dec 12;282:13-22. doi: 10.1016/j.neuroscience.2014.01.025. Epub 2014 Jan 23. Neuroscience. 2014. PMID: 24463000 Free PMC article. Review.
-
Dopamine D2 autoreceptor interactome: Targeting the receptor complex as a strategy for treatment of substance use disorder.Pharmacol Ther. 2020 Sep;213:107583. doi: 10.1016/j.pharmthera.2020.107583. Epub 2020 May 27. Pharmacol Ther. 2020. PMID: 32473160 Free PMC article. Review.
Cited by
-
D2R signaling in striatal spiny neurons modulates L-DOPA induced dyskinesia.iScience. 2022 Oct 4;25(10):105263. doi: 10.1016/j.isci.2022.105263. eCollection 2022 Oct 21. iScience. 2022. PMID: 36274959 Free PMC article.
-
D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum.Mol Psychiatry. 2023 Feb;28(2):625-638. doi: 10.1038/s41380-022-01809-y. Epub 2022 Oct 4. Mol Psychiatry. 2023. PMID: 36195641 Free PMC article.
-
Gi/o protein-coupled receptors in dopamine neurons inhibit the sodium leak channel NALCN.Elife. 2018 Dec 17;7:e40984. doi: 10.7554/eLife.40984. Elife. 2018. PMID: 30556810 Free PMC article.
-
Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons.Brain. 2014 Aug;137(Pt 8):2287-302. doi: 10.1093/brain/awu131. Epub 2014 Jun 16. Brain. 2014. PMID: 24934288 Free PMC article.
-
Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):E8178-E8186. doi: 10.1073/pnas.1614347113. Epub 2016 Dec 1. Proc Natl Acad Sci U S A. 2016. PMID: 27911814 Free PMC article.
References
-
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. - PubMed
-
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. - PubMed
-
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004a;42:653–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

