Chloroplast β-barrel proteins are assembled into the mitochondrial outer membrane in a process that depends on the TOM and TOB complexes
- PMID: 22745120
- PMCID: PMC3431683
- DOI: 10.1074/jbc.M112.382093
Chloroplast β-barrel proteins are assembled into the mitochondrial outer membrane in a process that depends on the TOM and TOB complexes
Abstract
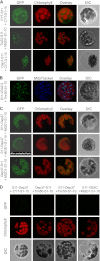
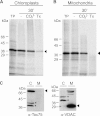
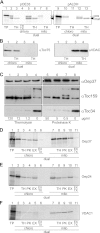
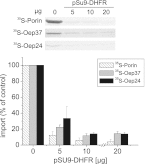
Membrane-embedded β-barrel proteins are found in the outer membranes (OM) of Gram-negative bacteria, mitochondria and chloroplasts. In eukaryotic cells, precursors of these proteins are synthesized in the cytosol and have to be sorted to their corresponding organelle. Currently, the signal that ensures their specific targeting to either mitochondria or chloroplasts is ill-defined. To address this issue, we studied targeting of the chloroplast β-barrel proteins Oep37 and Oep24. We found that both proteins can be integrated in vitro into isolated plant mitochondria. Furthermore, upon their expression in yeast cells Oep37 and Oep24 were exclusively located in the mitochondrial OM. Oep37 partially complemented the growth phenotype of yeast cells lacking Porin, the general metabolite transporter of this membrane. Similarly to mitochondrial β-barrel proteins, Oep37 and Oep24 expressed in yeast cells were assembled into the mitochondrial OM in a pathway dependent on the TOM and TOB complexes. Taken together, this study demonstrates that the central mitochondrial components that mediate the import of yeast β-barrel proteins can deal with precursors of chloroplast β-barrel proteins. This implies that the mitochondrial import machinery does not recognize signals that are unique to mitochondrial β-barrel proteins. Our results further suggest that dedicated targeting factors had to evolve in plant cells to prevent mis-sorting of chloroplast β-barrel proteins to mitochondria.
Figures






Similar articles
-
Identification of mitochondrial coenzyme a transporters from maize and Arabidopsis.Plant Physiol. 2013 Jun;162(2):581-8. doi: 10.1104/pp.113.218081. Epub 2013 Apr 16. Plant Physiol. 2013. PMID: 23590975 Free PMC article.
-
Yeast mitochondria can process de novo designed β-barrel proteins.FEBS J. 2024 Jan;291(2):292-307. doi: 10.1111/febs.16950. Epub 2023 Oct 2. FEBS J. 2024. PMID: 37723586
-
Role of phosphatidylethanolamine in the biogenesis of mitochondrial outer membrane proteins.J Biol Chem. 2013 Jun 7;288(23):16451-16459. doi: 10.1074/jbc.M112.442392. Epub 2013 Apr 26. J Biol Chem. 2013. PMID: 23625917 Free PMC article.
-
Biogenesis of beta-barrel proteins in evolutionary context.Int J Med Microbiol. 2015 Feb;305(2):259-64. doi: 10.1016/j.ijmm.2014.12.009. Epub 2014 Dec 24. Int J Med Microbiol. 2015. PMID: 25596888 Review.
-
Role of the TOM Complex in Protein Import into Mitochondria: Structural Views.Annu Rev Biochem. 2022 Jun 21;91:679-703. doi: 10.1146/annurev-biochem-032620-104527. Epub 2022 Mar 14. Annu Rev Biochem. 2022. PMID: 35287471 Review.
Cited by
-
Evolutionary conservation in biogenesis of β-barrel proteins allows mitochondria to assemble a functional bacterial trimeric autotransporter protein.J Biol Chem. 2014 Oct 24;289(43):29457-70. doi: 10.1074/jbc.M114.565655. Epub 2014 Sep 4. J Biol Chem. 2014. PMID: 25190806 Free PMC article.
-
The Biogenesis Process of VDAC - From Early Cytosolic Events to Its Final Membrane Integration.Front Physiol. 2021 Aug 12;12:732742. doi: 10.3389/fphys.2021.732742. eCollection 2021. Front Physiol. 2021. PMID: 34456757 Free PMC article. Review.
-
Bipartite Topology of Treponema pallidum Repeat Proteins C/D and I: OUTER MEMBRANE INSERTION, TRIMERIZATION, AND PORIN FUNCTION REQUIRE A C-TERMINAL β-BARREL DOMAIN.J Biol Chem. 2015 May 8;290(19):12313-31. doi: 10.1074/jbc.M114.629188. Epub 2015 Mar 24. J Biol Chem. 2015. PMID: 25805501 Free PMC article.
-
ATP-independent assembly machinery of bacterial outer membranes: BAM complex structure and function set the stage for next-generation therapeutics.Protein Sci. 2024 Feb;33(2):e4896. doi: 10.1002/pro.4896. Protein Sci. 2024. PMID: 38284489 Free PMC article. Review.
-
Sam37 is crucial for formation of the mitochondrial TOM-SAM supercomplex, thereby promoting β-barrel biogenesis.J Cell Biol. 2015 Sep 28;210(7):1047-54. doi: 10.1083/jcb.201504119. J Cell Biol. 2015. PMID: 26416958 Free PMC article.
References
-
- Goetze T. A., Philippar K., Ilkavets I., Soll J., Wagner R. (2006) OEP37 is a new member of the chloroplast outer membrane ion channels. J. Biol. Chem. 281, 17989–17998 - PubMed
-
- Hemmler R., Becker T., Schleiff E., Bölter B., Stahl T., Soll J., Götze T. A., Braams S., Wagner R. (2006) Molecular properties of Oep21, an ATP-regulated anion-selective solute channel from the outer chloroplast membrane. J. Biol. Chem. 281, 12020–12029 - PubMed
-
- Schleiff E., Maier U. G., Becker T. (2011) Omp85 in eukaryotic systems. One protein family with distinct functions. Biol. Chem. 392, 21–27 - PubMed
-
- Soll J., Schleiff E. (2004) Protein import into chloroplasts. Nat. Rev. Mol. Cell Biol. 5, 198–208 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

