Tight junctions on the move: molecular mechanisms for epithelial barrier regulation
- PMID: 22731710
- PMCID: PMC3690943
- DOI: 10.1111/j.1749-6632.2012.06613.x
Tight junctions on the move: molecular mechanisms for epithelial barrier regulation
Abstract
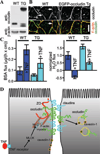
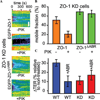
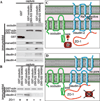
Increasing evidence suggests that the tight junction is a dynamically regulated structure. Cytoskeletal reorganization, particularly myosin light chain phosphorylation--induced actomyosin contraction, has increasingly been recognized as a mediator of physiological and pathophysiological tight junction regulation. However, our understanding of molecular mechanisms of tight junction modulation remains limited. Recent studies using live cell and live animal imaging techniques allowed us to peek into the molecular details of tight junction regulation. At resting conditions, the tight junction is maintained by dynamic protein-protein interactions, which may provide a platform for rapid tight junction regulation. Following stimulation, distinct forms of tight junction protein reorganization were observed. Tumor necrosis factor (TNF-α) causes a myosin light chain kinase (MLCK)--mediated barrier regulation by inducing occludin removal from the tight junction through caveolar endocytosis. In contrast, MLCK- and CK2-inhibition--caused tight junction regulation is mediated by altered zonula occludens (ZO)-1 protein dynamics and requires ZO-1--mediated protein-protein interaction, potentially through regulating claudin function. Although some of the molecular details are missing, studies summarized above point to modulating protein localization and dynamics that are common mechanisms for tight junction regulation.
© 2012 New York Academy of Sciences.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

Similar articles
-
Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo.J Cell Biol. 2010 Apr 5;189(1):111-26. doi: 10.1083/jcb.200902153. Epub 2010 Mar 29. J Cell Biol. 2010. PMID: 20351069 Free PMC article.
-
MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8237-41. doi: 10.1073/pnas.0908869107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404178 Free PMC article.
-
Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism.Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G308-18. doi: 10.1152/ajpgi.00582.2006. Epub 2007 Apr 19. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17446308
-
Tight junctions and the molecular basis for regulation of paracellular permeability.Am J Physiol. 1995 Oct;269(4 Pt 1):G467-75. doi: 10.1152/ajpgi.1995.269.4.G467. Am J Physiol. 1995. PMID: 7485497 Review.
-
Myosin light chain kinase: pulling the strings of epithelial tight junction function.Ann N Y Acad Sci. 2012 Jul;1258(1):34-42. doi: 10.1111/j.1749-6632.2012.06526.x. Ann N Y Acad Sci. 2012. PMID: 22731713 Free PMC article. Review.
Cited by
-
Connections matter--how viruses use cell–cell adhesion components.J Cell Sci. 2015 Feb 1;128(3):431-9. doi: 10.1242/jcs.159400. J Cell Sci. 2015. PMID: 26046138 Free PMC article. Review.
-
Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels.J Am Coll Surg. 2016 Jun;222(6):1009-17. doi: 10.1016/j.jamcollsurg.2015.12.006. Epub 2015 Dec 18. J Am Coll Surg. 2016. PMID: 27106638 Free PMC article.
-
Deficiency of Stomach-Type Claudin-18 in Mice Induces Gastric Tumor Formation Independent of H pylori Infection.Cell Mol Gastroenterol Hepatol. 2019;8(1):119-142. doi: 10.1016/j.jcmgh.2019.03.003. Epub 2019 Mar 23. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30910700 Free PMC article.
-
Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: role in barrier functions.Am J Physiol Cell Physiol. 2015 Jul 1;309(1):C38-50. doi: 10.1152/ajpcell.00388.2014. Epub 2015 May 6. Am J Physiol Cell Physiol. 2015. PMID: 25948735 Free PMC article.
-
Lactobacillus acidophilus inhibits the TNF-α-induced increase in intestinal epithelial tight junction permeability via a TLR-2 and PI3K-dependent inhibition of NF-κB activation.Front Immunol. 2024 Jul 16;15:1348010. doi: 10.3389/fimmu.2024.1348010. eCollection 2024. Front Immunol. 2024. PMID: 39081324 Free PMC article.
References
-
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. - PubMed
-
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. - PubMed
-
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. - PubMed
-
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

