Neuronal dysfunction with aging and its amelioration
- PMID: 22728441
- PMCID: PMC3410143
- DOI: 10.2183/pjab.88.266
Neuronal dysfunction with aging and its amelioration
Abstract
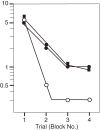
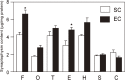
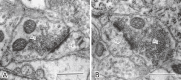
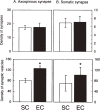
The author focused on the functional decline of synapses in the brain with aging to understand the underlying mechanisms and to ameliorate the deficits. The first attempt was to unravel the neuronal functions of gangliosides so that gangliosides could be used for enhancing synaptic activity. The second attempt was to elicit the neuronal plasticity in aged animals through enriched environmental stimulation and nutritional intervention. Environmental stimuli were revealed neurochemically and morphologically to develop synapses leading to enhanced cognitive function. Dietary restriction as a nutritional intervention restored the altered metabolism of neuronal membranes with aging, providing a possible explanation for the longevity effect of dietary restriction. These results obtained with aging and dementia models of animals would benefit aged people.
Figures

Similar articles
-
Molecular bases of caloric restriction regulation of neuronal synaptic plasticity.Mol Neurobiol. 2008 Oct;38(2):167-77. doi: 10.1007/s12035-008-8040-1. Epub 2008 Aug 30. Mol Neurobiol. 2008. PMID: 18759009 Review.
-
Caloric restriction and brain function.Curr Opin Clin Nutr Metab Care. 2008 Nov;11(6):686-92. doi: 10.1097/MCO.0b013e328313968f. Curr Opin Clin Nutr Metab Care. 2008. PMID: 18827571 Review.
-
Senescent synapses and hippocampal circuit dynamics.Trends Neurosci. 2010 Mar;33(3):153-61. doi: 10.1016/j.tins.2009.12.003. Epub 2010 Jan 12. Trends Neurosci. 2010. PMID: 20071039 Free PMC article. Review.
-
Bidirectional metabolic regulation of neurocognitive function.Neurobiol Learn Mem. 2011 Nov;96(4):507-16. doi: 10.1016/j.nlm.2011.01.004. Epub 2011 Jan 12. Neurobiol Learn Mem. 2011. PMID: 21236352 Free PMC article. Review.
-
Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice.Curr Biol. 2004 Nov 9;14(21):1907-15. doi: 10.1016/j.cub.2004.10.021. Curr Biol. 2004. PMID: 15530391
Cited by
-
Ganglioside biochemistry.ISRN Biochem. 2012 Dec 19;2012:506160. doi: 10.5402/2012/506160. eCollection 2012. ISRN Biochem. 2012. PMID: 25969757 Free PMC article. Review.
-
Elucidation of the enigma of glycosphingolipids in the regulation of inflammation and degeneration - Great progress over the last 70 years.Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(3):136-149. doi: 10.2183/pjab.95.011. Proc Jpn Acad Ser B Phys Biol Sci. 2019. PMID: 30853699 Free PMC article. Review.
-
Novel Molecular Mechanisms of Gangliosides in the Nervous System Elucidated by Genetic Engineering.Int J Mol Sci. 2020 Mar 11;21(6):1906. doi: 10.3390/ijms21061906. Int J Mol Sci. 2020. PMID: 32168753 Free PMC article. Review.
References
-
- Tanaka Y., Hasegawa A., Ando S. (1996) Impaired synaptic functions with aging as characterized by decreased calcium influx and acetylcholine release. J. Neurosci. Res. 43, 63–70 - PubMed
-
- Tanaka Y., Ando S. (1990) Synaptic aging as revealed by changes in membrane potential and decreased activity of Na+,K+-ATPase. Brain Res. 506, 46–52 - PubMed
-
- Ando S., Tanaka Y. (1990) Synaptic membrane aging in the central nervous system. Gerontology 36 (Supple. 1), 10–14 - PubMed
-
- Ando S., Tadenuma T., Tanaka Y., Fukui F., Kobayashi S., Ohashi Y., Kawabata T. (2001) Enhancement of learning activity and cholinergic synaptic function by carnitine in aging rats. J. Neurosci. Res. 66, 266–271 - PubMed
-
- Furuse H., Waki H., Kaneko K., Fujii S., Miura M., Sasaki H., Ito K.-I., Kato H., Ando S. (1998) Effect of the mono- and tetra-sialogangliosides, GM1 and GQ1b, on long-term potentiation in the CA1 hippocampal neurons of the guinea pig. Exp. Brain Res. 123, 307–314 - PubMed

