Immunochemistry of pathogenic yeast, Candida species, focusing on mannan
- PMID: 22728440
- PMCID: PMC3410142
- DOI: 10.2183/pjab.88.250
Immunochemistry of pathogenic yeast, Candida species, focusing on mannan
Abstract
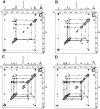
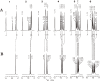
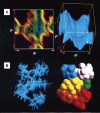
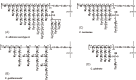
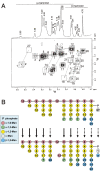
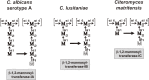
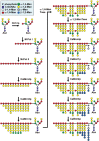
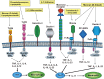
This review describes recent findings based on structural and immunochemical analyses of the cell wall mannan of Candida albicans, and other medically important Candida species. Mannan has been shown to consist of α-1,2-, α-1,3-, α-1,6-, and β-1,2-linked mannopyranose units with few phosphate groups. Each Candida species has a unique mannan structure biosynthesized by sequential collaboration between species-specific mannosyltransferases. In particular, the β-1,2-linked mannose units have been shown to comprise a characteristic oligomannosyl side chain that is strongly antigenic. For these pathogenic Candida species, cell-surface mannan was also found to participate in the adhesion to the epithelial cells, recognition by innate immune receptors and development of pathogenicity. Therefore, clarification of the precise chemical structure of Candida mannan is indispensable for understanding the mechanism of pathogenicity, and for development of new antifungal drugs and immunotherapeutic procedures.
Figures


Similar articles
-
Structures of cell wall mannans of pathogenic Candida tropicalis IFO 0199 and IFO 1647 yeast strains.Infect Immun. 1994 Feb;62(2):615-22. doi: 10.1128/iai.62.2.615-622.1994. Infect Immun. 1994. PMID: 7507898 Free PMC article.
-
Structure of a cell wall mannan from the pathogenic yeast, Candida catenulata: assignment of 1H nuclear magnetic resonance chemical shifts of the inner alpha-1,6-linked mannose residues substituted by a side chain.Arch Biochem Biophys. 1997 May 1;341(1):70-4. doi: 10.1006/abbi.1997.9939. Arch Biochem Biophys. 1997. PMID: 9143354
-
Structure and antigenicity of the mannans of Candida famata and Candida saitoana: comparative study with the mannan of Candida guilliermondii.Arch Biochem Biophys. 1996 Dec 1;336(1):49-58. doi: 10.1006/abbi.1996.0531. Arch Biochem Biophys. 1996. PMID: 8951034
-
[Immunochemical study on mannan, the antigenic polysaccharide of pathogenic yeasts in man of genus Candida].Yakugaku Zasshi. 1995 Apr;115(4):280-94. doi: 10.1248/yakushi1947.115.4_280. Yakugaku Zasshi. 1995. PMID: 7541458 Review. Japanese.
-
Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34.Curr Top Med Mycol. 1997 Dec;8(1-2):57-70. Curr Top Med Mycol. 1997. PMID: 9504067 Review.
Cited by
-
Potential of Chemically Synthesized Oligosaccharides To Define the Carbohydrate Moieties of the Fungal Cell Wall Responsible for the Human Immune Response, Using Aspergillus fumigatus Galactomannan as a Model.mSphere. 2020 Jan 8;5(1):e00688-19. doi: 10.1128/mSphere.00688-19. mSphere. 2020. PMID: 31915215 Free PMC article.
-
Pathogenesis of Dermatophytosis: Sensing the Host Tissue.Mycopathologia. 2017 Feb;182(1-2):215-227. doi: 10.1007/s11046-016-0057-9. Epub 2016 Sep 2. Mycopathologia. 2017. PMID: 27590362 Review.
-
Effects of yeast culture on growth performance, immune function, antioxidant capacity and hormonal profile in Mongolian ram lambs.Front Vet Sci. 2024 Jul 23;11:1424073. doi: 10.3389/fvets.2024.1424073. eCollection 2024. Front Vet Sci. 2024. PMID: 39109341 Free PMC article.
-
Select Streptococci Can Degrade Candida Mannan To Facilitate Growth.Appl Environ Microbiol. 2022 Feb 22;88(4):e0223721. doi: 10.1128/aem.02237-21. Epub 2021 Dec 22. Appl Environ Microbiol. 2022. PMID: 34936835 Free PMC article.
-
A new tool to quantify receptor recruitment to cell contact sites during host-pathogen interaction.PLoS Comput Biol. 2014 May 29;10(5):e1003639. doi: 10.1371/journal.pcbi.1003639. eCollection 2014 May. PLoS Comput Biol. 2014. PMID: 24874253 Free PMC article.
References
-
- Pfaller M.A. (1996) Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22, S89–S94 - PubMed
-
- Tsuchiya T., Fukazawa Y., Kawakita S. (1965) Significance of serological studies on yeast. Mycopathol. Mycol. Appl. 26, 1–15 - PubMed
-
- Tsuchiya T., Fukazawa Y., Taguchi M., Nakase T., Shinoda T. (1974) Serologic aspects on yeast classification. Mycopathol. Mycol. Appl. 53, 77–91 - PubMed
-
- Tsuchiya, T., Taguchi, S., Fukazawa, Y. and Shinoda, T. (1984) Serological characterization of yeasts as an aid in identification and classification. In Methods in Microbiology (ed. Bergan, T.). Academic Press, New York, 16, pp. 75–126.
-
- Fukazawa, Y., Nishikawa, A., Suzuki, M. and Shinoda, T. (1980) Immunochemical basis of the serologic specificity of the yeast: Immunochemical determinants of several antigenic factors of yeasts. In Medical Mycology (ed. Preusser, H.J.) Zbl. Bakt. Suppl. 8. Gustav Fischer Verlag, Stuttgart, New York, pp. 126–136.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

