Proteasome protease mediated regulation of cytokine induction and inflammation
- PMID: 22728331
- PMCID: PMC3465503
- DOI: 10.1016/j.bbamcr.2012.06.016
Proteasome protease mediated regulation of cytokine induction and inflammation
Abstract
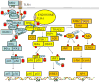
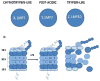
We have previously demonstrated that proteasome serves as a central regulator of inflammation and macrophage function. Until recently, proteasomes have generally been considered to play a relatively passive role in the regulation of cellular activity, i.e., any ubiquitinated protein was considered to be in discriminatively targeted for degradation by the proteasome. We have demonstrated, however, by using specific proteasome protease inhibitors and knockout mice lacking specific components of immunoproteasomes, that proteasomes (containing X, Y, and Z protease subunits) and immunoproteasomes (containing LMP7, LMP2, and LMP10 protease subunits) have well-defined functions in cytokine induction and inflammation based on their individual protease activities. We have also shown that LPS-TLR mediated signaling in the murine RAW 264.7 macrophage cell line results in the replacement of macrophage immunoproteasomal subunits. Such modifications serve as pivotal regulators of LPS-induced inflammation. Our findings support the relatively novel concept that defects in structure/function of proteasome protease subunits caused by genetic disorders, aging, diet, or drugs may well have the potential to contribute to modulation of proteasome activity. Of particular relevance, we have identified quercetin and resveratrol, significant constituents present in berries and in red wine respectively, as two novel proteasome inhibitors that have been previously implicated as disease-modifying natural products. We posit that natural proteasome inhibitors/activators can potentially be used as therapeutic response modifiers to prevent/treat diseases through pathways involving the ubiquitin-proteasome pathway (UP-pathway), which likely functions as a master regulator involved in control of overall inflammatory responses. This article is part of a Special Issue entitled: Ubiquitin Drug Discovery and Diagnostics.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models.Lipids Health Dis. 2011 Oct 12;10:177. doi: 10.1186/1476-511X-10-177. Lipids Health Dis. 2011. PMID: 21992595 Free PMC article.
-
The immunoproteasomes regulate LPS-induced TRIF/TRAM signaling pathway in murine macrophages.Cell Biochem Biophys. 2011 Jun;60(1-2):119-26. doi: 10.1007/s12013-011-9183-7. Cell Biochem Biophys. 2011. PMID: 21455681 Free PMC article.
-
LPS-induced formation of immunoproteasomes: TNF-α and nitric oxide production are regulated by altered composition of proteasome-active sites.Cell Biochem Biophys. 2011 Jun;60(1-2):77-88. doi: 10.1007/s12013-011-9182-8. Cell Biochem Biophys. 2011. PMID: 21455682 Free PMC article.
-
Dysregulation of immunoproteasomes in autoinflammatory syndromes.Int Immunol. 2019 Sep 18;31(10):631-637. doi: 10.1093/intimm/dxy059. Int Immunol. 2019. PMID: 30169676 Review.
-
Interferon-gamma inducible exchanges of 20S proteasome active site subunits: why?Biochimie. 2001 Mar-Apr;83(3-4):367-72. doi: 10.1016/s0300-9084(01)01251-2. Biochimie. 2001. PMID: 11295499 Review.
Cited by
-
Label-free Proteomic Analysis of Exosomes Derived from Inducible Hepatitis B Virus-Replicating HepAD38 Cell Line.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S144-S160. doi: 10.1074/mcp.M116.063503. Epub 2017 Feb 27. Mol Cell Proteomics. 2017. PMID: 28242843 Free PMC article.
-
The lung is protected from spontaneous inflammation by autophagy in myeloid cells.J Immunol. 2015 Jun 1;194(11):5465-71. doi: 10.4049/jimmunol.1403249. Epub 2015 Apr 24. J Immunol. 2015. PMID: 25911758 Free PMC article.
-
The Constitutive Extracellular Protein Release by Acute Myeloid Leukemia Cells-A Proteomic Study of Patient Heterogeneity and Its Modulation by Mesenchymal Stromal Cells.Cancers (Basel). 2021 Mar 25;13(7):1509. doi: 10.3390/cancers13071509. Cancers (Basel). 2021. PMID: 33806032 Free PMC article.
-
Dysregulation of Gene Expression of Key Signaling Mediators in PBMCs from People with Type 2 Diabetes Mellitus.Int J Mol Sci. 2023 Feb 1;24(3):2732. doi: 10.3390/ijms24032732. Int J Mol Sci. 2023. PMID: 36769056 Free PMC article.
-
Current understanding on the role of standard and immunoproteasomes in inflammatory/immunological pathways of multiple sclerosis.Autoimmune Dis. 2014;2014:739705. doi: 10.1155/2014/739705. Epub 2014 Jan 2. Autoimmune Dis. 2014. PMID: 24523959 Free PMC article. Review.
References
-
- Qureshi N, Vogel SN, Van Way C, III, Papasian CJ, Qureshi AA, Morrison DC. The proteasome. A central regulator of Inflammation and macrophage function. Immunol Res. 2005;31:243–260. - PubMed
-
- Qureshi N, Perera P-Y, Splitter G, Morrison DC, Vogel SN. The Proteasome as a LPS-binding protein in macrophages. Toxic lipopolysaccharide activates the proteasome complex. J Immunol. 2003;171:1515–1525. - PubMed
-
- Shen J, Reis J, Morrison DC, Papasian C, Raghavaikaimal S, Kolbert C, Qureshi AA, Vogel SN, Qureshi N. Key inflammatory signaling pathways are regulated by the proteasome. Shock. 2006;25:472–484. - PubMed
-
- Shen J, Gao JJ, Zhang G, Tan X, Morrison DC, Papasian CJ, Vogel SN, Qureshi N. Proteasome inhibitor, lactacystin blocks CpG DNA- and peptidoglycan induced inflammatory genes, cytokines and mitogen-activated protein kinases in macrophages. Shock. 2006;25:594–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

