Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells
- PMID: 22727020
- PMCID: PMC3425332
- DOI: 10.1186/1742-2094-9-138
Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells
Abstract
Background: Human immunodeficiency virus type 1 (HIV-1) induces neuronal dysfunction through host cellular factors and viral proteins including viral protein R (Vpr) released from infected macrophages/microglia. Vpr is important for infection of terminally differentiated cells such as macrophages. The objective of this study was to assess the effect of Vpr in the context of infectious virus particles on neuronal death through proinflammatory cytokines released from macrophages.
Methods: Monocyte-derived macrophages (MDM) were infected with either HIV-1 wild type (HIV-1wt), Vpr deleted mutant (HIV-1∆Vpr) or mock. Cell lysates and culture supernatants from MDMs were analyzed for the expression and release of proinflammatory cytokines by quantitative reverse transcription-PCR and enzyme-linked immunosorbent assay respectively. Mitogen-activated protein kinases (MAPK) were analyzed in activated MDMs by western blots. Further, the effect of Vpr on neuronal apoptosis was examined using primary neurons exposed to culture supernatants from HIV-1wt, HIV-1∆Vpr or mock-infected MDMs by Annexin-V staining, MTT and Caspase - Glo® 3/7 assays. The role of interleukin (IL)-1β, IL-8 and tumor necrosis factor (TNF)-α on neuronal apoptosis was also evaluated in the presence or absence of neutralizing antibodies against these cytokines.
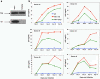
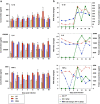
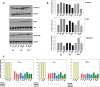
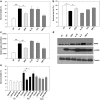
Results: HIV-1∆Vpr-infected MDMs exhibited reduced infection over time and specifically a significant downregulation of IL-1β, IL-8 and TNF-α at the transcriptional and/or protein levels compared to HIV-1wt-infected cultures. This downregulation was due to impaired activation of p38 and stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) in HIV-1∆Vpr-infected MDMs. The association of SAPK/JNK and p38 to IL-1β and IL-8 production was confirmed by blocking MAPKs that prevented the elevation of IL-1β and IL-8 in HIV-1wt more than in HIV-1∆Vpr-infected cultures. Supernatants from HIV-1∆Vpr-infected MDMs containing lower concentrations of IL-1β, IL-8 and TNF-α as well as viral proteins showed a reduced neurotoxicity compared to HIV-1wt-infected MDM supernatants. Reduction of neuronal death in the presence of anti-IL-1β and anti-IL-8 antibodies only in HIV-1wt-infected culture implies that the effect of Vpr on neuronal death is in part mediated through released proinflammatory factors.
Conclusion: Collectively, these results demonstrate the ability of HIV-1∆Vpr to restrict neuronal apoptosis through dysregulation of multiple proinflammatory cytokines in the infected target cells either directly or indirectly by suppressing viral replication.
Figures

Similar articles
-
Defining the roles for Vpr in HIV-1-associated neuropathogenesis.J Neurovirol. 2016 Aug;22(4):403-15. doi: 10.1007/s13365-016-0436-5. Epub 2016 Apr 7. J Neurovirol. 2016. PMID: 27056720 Free PMC article. Review.
-
HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R.J Neuroimmunol. 2008 Aug 30;200(1-2):100-10. doi: 10.1016/j.jneuroim.2008.06.015. Epub 2008 Jul 23. J Neuroimmunol. 2008. PMID: 18653246 Free PMC article.
-
HIV-1 Vpr-Induced Proinflammatory Response and Apoptosis Are Mediated through the Sur1-Trpm4 Channel in Astrocytes.mBio. 2020 Dec 8;11(6):e02939-20. doi: 10.1128/mBio.02939-20. mBio. 2020. PMID: 33293383 Free PMC article.
-
HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: implications for HIV-1 Associated Neuroinflammation.J Neuroimmune Pharmacol. 2017 Jun;12(2):233-248. doi: 10.1007/s11481-016-9708-3. Epub 2016 Oct 10. J Neuroimmune Pharmacol. 2017. PMID: 27726055
-
Macrophage signaling in HIV-1 infection.Retrovirology. 2010 Apr 9;7:34. doi: 10.1186/1742-4690-7-34. Retrovirology. 2010. PMID: 20380698 Free PMC article. Review.
Cited by
-
New Potential Axes of HIV Neuropathogenesis with Relevance to Biomarkers and Treatment.Curr Top Behav Neurosci. 2021;50:3-39. doi: 10.1007/7854_2019_126. Curr Top Behav Neurosci. 2021. PMID: 32040843
-
Peroxisome Proliferator-Activated Receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model.Sci Rep. 2019 Jul 1;9(1):9428. doi: 10.1038/s41598-019-45878-6. Sci Rep. 2019. PMID: 31263138 Free PMC article.
-
An Overview of Human Immunodeficiency Virus Type 1-Associated Common Neurological Complications: Does Aging Pose a Challenge?J Alzheimers Dis. 2017;60(s1):S169-S193. doi: 10.3233/JAD-170473. J Alzheimers Dis. 2017. PMID: 28800335 Free PMC article. Review.
-
Alzheimer's disease-like perturbations in HIV-mediated neuronal dysfunctions: understanding mechanisms and developing therapeutic strategies.Open Biol. 2020 Dec;10(12):200286. doi: 10.1098/rsob.200286. Epub 2020 Dec 23. Open Biol. 2020. PMID: 33352062 Free PMC article. Review.
-
Defining the roles for Vpr in HIV-1-associated neuropathogenesis.J Neurovirol. 2016 Aug;22(4):403-15. doi: 10.1007/s13365-016-0436-5. Epub 2016 Apr 7. J Neurovirol. 2016. PMID: 27056720 Free PMC article. Review.
References
-
- Ho DD, Sarngadharan MG, Resnick L, Dimarzoveronese F, Rota TR, Hirsch MS. Primary human T-lymphotropic virus type III infection. Ann Intern Med. 1985;103:880–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

