FGF inhibition directs BMP4-mediated differentiation of human embryonic stem cells to syncytiotrophoblast
- PMID: 22724507
- PMCID: PMC3475151
- DOI: 10.1089/scd.2012.0099
FGF inhibition directs BMP4-mediated differentiation of human embryonic stem cells to syncytiotrophoblast
Abstract
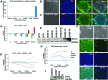
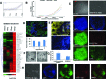
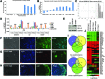
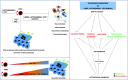
Bone morphogenetic protein (BMP) signaling is known to support differentiation of human embryonic stem cells (hESCs) into mesoderm and extraembryonic lineages, whereas other signaling pathways can largely influence this lineage specification. Here, we set out to reinvestigate the influence of ACTIVIN/NODAL and fibroblast growth factor (FGF) pathways on the lineage choices made by hESCs during BMP4-driven differentiation. We show that BMP activation, coupled with inhibition of both ACTIVIN/NODAL and FGF signaling, induces differentiation of hESCs, specifically to βhCG hormone-secreting multinucleated syncytiotrophoblast and does not support induction of embryonic and extraembryonic lineages, extravillous trophoblast, and primitive endoderm. It has been previously reported that FGF2 can switch BMP4-induced hESC differentiation outcome to mesendoderm. Here, we show that FGF inhibition alone, or in combination with either ACTIVIN/NODAL inhibition or BMP activation, supports hESC differentiation to hCG-secreting syncytiotrophoblast. We show that the inhibition of the FGF pathway acts as a key in directing BMP4-mediated hESC differentiation to syncytiotrophoblast.
Figures



Similar articles
-
BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages.Cell Stem Cell. 2011 Aug 5;9(2):144-55. doi: 10.1016/j.stem.2011.06.015. Cell Stem Cell. 2011. PMID: 21816365 Free PMC article.
-
Patterning of mouse embryonic stem cell-derived pan-mesoderm by Activin A/Nodal and Bmp4 signaling requires Fibroblast Growth Factor activity.Differentiation. 2008 Sep;76(7):745-59. doi: 10.1111/j.1432-0436.2007.00257.x. Epub 2008 Jan 3. Differentiation. 2008. PMID: 18177426
-
Optimizing bone morphogenic protein 4-mediated human embryonic stem cell differentiation into trophoblast-like cells using fibroblast growth factor 2 and transforming growth factor-β/activin/nodal signalling inhibition.Reprod Biomed Online. 2017 Sep;35(3):253-263. doi: 10.1016/j.rbmo.2017.06.003. Epub 2017 Jun 12. Reprod Biomed Online. 2017. PMID: 28647356
-
Signaling pathways during maintenance and definitive endoderm differentiation of embryonic stem cells.Int J Dev Biol. 2013;57(1):1-12. doi: 10.1387/ijdb.120115ls. Int J Dev Biol. 2013. PMID: 23585347 Review.
-
The role of activin/nodal and Wnt signaling in endoderm formation.Vitam Horm. 2011;85:207-16. doi: 10.1016/B978-0-12-385961-7.00010-X. Vitam Horm. 2011. PMID: 21353882 Review.
Cited by
-
Directed Differentiation of Human Pluripotent Stem Cells to Cytotrophoblast and Syncytiotrophoblast.Methods Mol Biol. 2024;2767:175-188. doi: 10.1007/7651_2022_469. Methods Mol Biol. 2024. PMID: 36773273
-
Highly efficient generation of self-renewing trophoblast from human pluripotent stem cells.iScience. 2024 Sep 3;27(10):110874. doi: 10.1016/j.isci.2024.110874. eCollection 2024 Oct 18. iScience. 2024. PMID: 39386760 Free PMC article.
-
Comparison of Four Protocols for In Vitro Differentiation of Human Embryonic Stem Cells into Trophoblast Lineages by BMP4 and Dual Inhibition of Activin/Nodal and FGF2 Signaling.Reprod Sci. 2024 Jan;31(1):173-189. doi: 10.1007/s43032-023-01334-5. Epub 2023 Sep 1. Reprod Sci. 2024. PMID: 37658178 Free PMC article.
-
Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):E1212-21. doi: 10.1073/pnas.1303094110. Epub 2013 Mar 14. Proc Natl Acad Sci U S A. 2013. PMID: 23493551 Free PMC article.
-
Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease.Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):E3882-91. doi: 10.1073/pnas.1604747113. Epub 2016 Jun 20. Proc Natl Acad Sci U S A. 2016. PMID: 27325764 Free PMC article.
References
-
- Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Xu RH. Chen X. Li DS. Li R. Addicks GC. Glennon C. Zwaka TP. Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. - PubMed
-
- Amit M. Shariki C. Margulets V. Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. - PubMed
-
- Beattie GM. Lopez AD. Bucay N. Hinton A. Firpo MT. King CC. Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. - PubMed
-
- Greber B. Lehrach H. Adjaye J. Control of early fate decisions in human ES cells by distinct states of TGFbeta pathway activity. Stem Cells Dev. 2008;17:1065–1077. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

