Viral CNS infections: role of glial pattern recognition receptors in neuroinflammation
- PMID: 22723794
- PMCID: PMC3379540
- DOI: 10.3389/fmicb.2012.00201
Viral CNS infections: role of glial pattern recognition receptors in neuroinflammation
Abstract
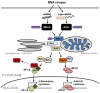
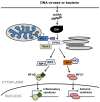
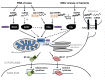
Viruses are the major causative agents of central nervous system (CNS) infection worldwide. RNA and DNA viruses trigger broad activation of glial cells including microglia and astrocytes, eliciting the release of an array of mediators that can promote innate and adaptive immune responses. Such responses can limit viral replication and dissemination leading to infection resolution. However, a defining feature of viral CNS infection is the rapid onset of severe neuroinflammation and overzealous glial responses are associated with significant neurological damage or even death. The mechanisms by which microglia and astrocytes perceive neurotropic RNA and DNA viruses are only now becoming apparent with the discovery of a variety of cell surface and cytosolic molecules that serve as sensors for viral components. In this review we discuss the role played by members of the Toll-like family of pattern recognition receptors (PRRs) in the inflammatory responses of glial cells to the principle causative agents of viral encephalitis. Importantly, we also describe the evidence for the involvement of a number of newly described intracellular PRRs, including retinoic acid-inducible gene I and DNA-dependent activator of IFN regulatory factors, that are thought to function as intracellular sensors of RNA and DNA viruses, respectively. Finally, we explore the possibility that cross-talk exists between these disparate viral sensors and their signaling pathways, and describe how glial cytosolic and cell surface/endosomal PRRs could act in a cooperative manner to promote the fulminant inflammation associated with acute neurotropic viral infection.
Keywords: RLR; TLR; astrocytes; microglia; neuroinflammation; pattern recognition receptors; viral encephalitis.
Figures
Similar articles
-
Cytosolic DNA Sensors and CNS Responses to Viral Pathogens.Front Cell Infect Microbiol. 2020 Sep 16;10:576263. doi: 10.3389/fcimb.2020.576263. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33042875 Free PMC article. Review.
-
RIG-I is required for VSV-induced cytokine production by murine glia and acts in combination with DAI to initiate responses to HSV-1.Glia. 2015 Dec;63(12):2168-80. doi: 10.1002/glia.22883. Epub 2015 Jul 3. Glia. 2015. PMID: 26146945 Free PMC article.
-
Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases.Brain Behav Immun. 2021 Jan;91:740-755. doi: 10.1016/j.bbi.2020.10.007. Epub 2020 Oct 8. Brain Behav Immun. 2021. PMID: 33039660 Free PMC article. Review.
-
Retinoic acid inducible gene-I mediated detection of bacterial nucleic acids in human microglial cells.J Neuroinflammation. 2020 May 1;17(1):139. doi: 10.1186/s12974-020-01817-1. J Neuroinflammation. 2020. PMID: 32357908 Free PMC article.
-
Cytosolic DNA sensors and glial responses to endogenous DNA.Front Immunol. 2023 Mar 14;14:1130172. doi: 10.3389/fimmu.2023.1130172. eCollection 2023. Front Immunol. 2023. PMID: 36999037 Free PMC article. Review.
Cited by
-
Insight into the divergent role of TRAIL in non-neoplastic neurological diseases.J Cell Mol Med. 2020 Oct;24(19):11070-11083. doi: 10.1111/jcmm.15757. Epub 2020 Aug 22. J Cell Mol Med. 2020. PMID: 32827246 Free PMC article. Review.
-
Cytosolic DNA Sensors and CNS Responses to Viral Pathogens.Front Cell Infect Microbiol. 2020 Sep 16;10:576263. doi: 10.3389/fcimb.2020.576263. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33042875 Free PMC article. Review.
-
Myd88 Initiates Early Innate Immune Responses and Promotes CD4 T Cells during Coronavirus Encephalomyelitis.J Virol. 2015 Sep;89(18):9299-312. doi: 10.1128/JVI.01199-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136579 Free PMC article.
-
SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia.Front Immunol. 2021 Apr 14;12:656700. doi: 10.3389/fimmu.2021.656700. eCollection 2021. Front Immunol. 2021. PMID: 33936086 Free PMC article.
-
Murine astrocytes are responsive to the pro-inflammatory effects of IL-20.Neurosci Lett. 2019 Aug 24;708:134334. doi: 10.1016/j.neulet.2019.134334. Epub 2019 Jun 22. Neurosci Lett. 2019. PMID: 31238130 Free PMC article.
References
-
- Aloisi F., Penna G., Cerase J., Menendez Iglesias B., Adorini L. (1997). IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 159 1604–1612 - PubMed
-
- Aravalli R. N., Hu S., Rowen T. N., Gekker G., Lokensgard J. R. (2006). Differential apoptotic signaling in primary glial cells infected with herpes simplex virus 1. J. Neurovirol. 12 501–510 - PubMed
-
- Baringer J. R. (2008). Herpes simplex infections of the nervous system. Neurol. Clin. 26 657–674 - PubMed
-
- Bauer J., Huitinga I., Zhao W., Lassmann H., Hickey W. F., Dijkstra C. D. (1995). The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia 15 437–446 - PubMed
LinkOut - more resources
Full Text Sources

