Cannabinoid receptor-mediated regulation of neuronal activity and signaling in glomeruli of the main olfactory bulb
- PMID: 22723687
- PMCID: PMC3397690
- DOI: 10.1523/JNEUROSCI.5333-11.2012
Cannabinoid receptor-mediated regulation of neuronal activity and signaling in glomeruli of the main olfactory bulb
Abstract
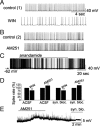
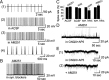
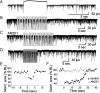
Cannabinoid receptors (CB1Rs) are present in glomeruli of the main olfactory bulb. The functions of CB1Rs and their endogenous activators, endocannabinoids, for glomerular signaling are unknown. Glomeruli contain at least three types of neurons: periglomerular (PG), external tufted (ET), and short-axon (SA) cells. PG cells form inhibitory GABAergic dendrodendritic synapses with ET cells. ET cells form excitatory glutamatergic dendrodendritic synapses with PG and SA cells. In mouse brain slices, we used whole-cell patch-clamp recordings to study the role of CB1Rs in regulating PG and ET cells. Cannabinoids displayed strong, direct inhibitory effects on PG cells and weak effects on ET cells. Single pulses or a train of pulses of depolarizing current injected into an ET cell evoked suppression of IPSCs. This suggests retrograde endocannabinoid signaling, namely, depolarization-induced suppression of inhibition (DSI) in ET cells. Our results support the hypothesis that burst firing of ET cells triggers the release of endocannabinoids which in turn directly inhibit PG cells and reduce GABA release from PG cells. This, in turn, can result in a transient reduction of PG cell inhibitory input to ET cells.
Figures
Similar articles
-
Depolarization-induced release of endocannabinoids by murine dorsal motor nucleus of the vagus nerve neurons differentially regulates inhibitory and excitatory neurotransmission.Neuropharmacology. 2009 Jun;56(8):1106-15. doi: 10.1016/j.neuropharm.2009.03.009. Epub 2009 Mar 28. Neuropharmacology. 2009. PMID: 19332082
-
Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses.Nat Neurosci. 2010 May;13(5):592-600. doi: 10.1038/nn.2517. Epub 2010 Mar 28. Nat Neurosci. 2010. PMID: 20348918 Free PMC article.
-
Endocannabinoid- and mGluR5-dependent short-term synaptic depression in an isolated neuron/bouton preparation from the hippocampal CA1 region.J Neurophysiol. 2008 Aug;100(2):1041-52. doi: 10.1152/jn.90226.2008. Epub 2008 May 21. J Neurophysiol. 2008. PMID: 18497350 Free PMC article.
-
[Cannabinoid applications in glaucoma].Arch Soc Esp Oftalmol. 2011 Jan;86(1):16-23. doi: 10.1016/j.oftal.2010.11.015. Epub 2011 Feb 24. Arch Soc Esp Oftalmol. 2011. PMID: 21414525 Review. Spanish.
-
Deciphering the mechanisms of reciprocal regulation or interdependence at the cannabinoid CB1 receptors and cyclooxygenase-2 level: Effects on mood, cognitive implications, and synaptic signaling.Neurosci Biobehav Rev. 2023 Dec;155:105439. doi: 10.1016/j.neubiorev.2023.105439. Epub 2023 Oct 28. Neurosci Biobehav Rev. 2023. PMID: 37898448 Review.
Cited by
-
Epigenetic Effects of Drugs of Abuse.Int J Environ Res Public Health. 2018 Sep 25;15(10):2098. doi: 10.3390/ijerph15102098. Int J Environ Res Public Health. 2018. PMID: 30257440 Free PMC article. Review.
-
Cannabinoid Receptors Modulate Excitation of an Olfactory Bulb Local Circuit by Cortical Feedback.Front Cell Neurosci. 2018 Mar 2;12:47. doi: 10.3389/fncel.2018.00047. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29551963 Free PMC article.
-
Cannabinoid Control of Olfactory Processes: The Where Matters.Genes (Basel). 2020 Apr 16;11(4):431. doi: 10.3390/genes11040431. Genes (Basel). 2020. PMID: 32316252 Free PMC article. Review.
-
Cannabinoid receptor-mediated modulation of inhibitory inputs to mitral cells in the main olfactory bulb.J Neurophysiol. 2019 Aug 1;122(2):749-759. doi: 10.1152/jn.00100.2018. Epub 2019 Jun 19. J Neurophysiol. 2019. PMID: 31215302 Free PMC article.
-
Olfactory connectivity mediates sleep-dependent food choices in humans.Elife. 2019 Oct 8;8:e49053. doi: 10.7554/eLife.49053. Elife. 2019. PMID: 31591965 Free PMC article.
References
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
-
- Allen Institute for Brain Science. Allen mouse brain atlas [Online] Seattle. 2009. http://mouse.brain-map.org.
-
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. - PubMed
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. - PubMed
-
- Ennis M, Hamilton KA, Hayar A. Neurochemistry of the main olfactory system. In: Johnson DA, Lajtha A, editors. Handbook of neurochemistry and molecular neurobiology. Springer: Heidelberg; 2007. pp. 137–204. vol: Sensory neurochemistry.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

