Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells
- PMID: 22717603
- PMCID: PMC3439602
- DOI: 10.1016/j.molonc.2012.05.002
Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells
Abstract
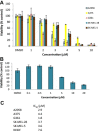
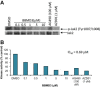
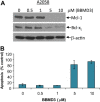
Persistent Jak/Stat3 signal transduction plays a crucial role in tumorigenesis and immune development. Activated Jak/Stat3 signaling has been validated as a promising molecular target for cancer therapeutics discovery and development. Berbamine (BBM), a natural bis-benzylisoquinoline alkaloid, was identified from the traditional Chinese herbal medicine Berberis amurensis used for treatment of cancer patients. While BBM has been shown to have potent antitumor activities with low toxicity in various cancer types, the molecular mechanism of action of BBM remains largely unknown. Here, we determine the antitumor activities of 13 synthetic berbamine derivatives (BBMDs) against human solid tumor cells. BBMD3, which is the most potent in this series of novel BBMDs, exhibits over 6-fold increase in biological activity compared to natural BBM. Moreover, BBMD3, directly inhibits Jak2 autophosphorylation kinase activity in vitro with IC(50)0.69 μM. Autophosphorylation of Jak2 kinase at Tyr1007/1008 sites also was strongly inhibited in the range of 15 μM of BBMD3 in human melanoma cells at 4h after treatment. Following inhibition of autophosphorylation of Jak2, BBMD3 blocked constitutive activation of downstream Stat3 signaling in melanoma cells. BBMD3 also down-regulated expression of the Stat3 target proteins Mcl-1and Bcl-x(L), associated with induction of apoptosis. In sum, our findings demonstrate that the novel berbamine derivative BBMD3 is an inhibitor of the Jak2/Stat3 signaling pathway, providing evidence for a molecular mechanism whereby BBMD3 exerts at least in part the apoptosis of human melanoma cells. In addition, BBMD3 represents a promising lead compound for development of new therapeutics for cancer treatment.
Copyright © 2012 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
A novel synthetic derivative of the natural product berbamine inhibits cell viability and induces apoptosis of human osteosarcoma cells, associated with activation of JNK/AP-1 signaling.Cancer Biol Ther. 2013 Nov;14(11):1024-31. doi: 10.4161/cbt.26045. Epub 2013 Aug 28. Cancer Biol Ther. 2013. PMID: 24025361 Free PMC article.
-
A novel berbamine derivative inhibits cell viability and induces apoptosis in cancer stem-like cells of human glioblastoma, via up-regulation of miRNA-4284 and JNK/AP-1 signaling.PLoS One. 2014 Apr 14;9(4):e94443. doi: 10.1371/journal.pone.0094443. eCollection 2014. PLoS One. 2014. PMID: 24732116 Free PMC article.
-
Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway.Toxicology. 2013 Feb 8;304:120-31. doi: 10.1016/j.tox.2012.12.018. Epub 2013 Jan 8. Toxicology. 2013. PMID: 23313376
-
Regulation of Cell-Signaling Pathways by Berbamine in Different Cancers.Int J Mol Sci. 2022 Mar 2;23(5):2758. doi: 10.3390/ijms23052758. Int J Mol Sci. 2022. PMID: 35269900 Free PMC article. Review.
-
Targeting JAK2/STAT3 for the treatment of cancer: A review on recent advancements in molecular development using structural analysis and SAR investigations.Bioorg Chem. 2024 Feb;143:107095. doi: 10.1016/j.bioorg.2023.107095. Epub 2024 Jan 4. Bioorg Chem. 2024. PMID: 38211548 Review.
Cited by
-
Novel harmine derivatives for tumor targeted therapy.Oncotarget. 2015 Apr 20;6(11):8988-9001. doi: 10.18632/oncotarget.3276. Oncotarget. 2015. PMID: 25940702 Free PMC article.
-
Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway.Int J Clin Exp Pathol. 2015 Jan 1;8(1):615-21. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25755753 Free PMC article.
-
Lentiginoses in polycythemia vera patient: Is there a role for JAK2 (V617F) mutation?JAKSTAT. 2015 Jul 24;4(1):e1071000. doi: 10.1080/21623996.2015.1071000. eCollection 2015. JAKSTAT. 2015. PMID: 26413426 Free PMC article.
-
Berbamine Inhibits Cell Proliferation and Migration and Induces Cell Death of Lung Cancer Cells via Regulating c-Maf, PI3K/Akt, and MDM2-P53 Pathways.Evid Based Complement Alternat Med. 2021 Jul 8;2021:5517143. doi: 10.1155/2021/5517143. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34306137 Free PMC article.
-
MiR362-3p Alleviates Osteosarcoma by Regulating the IL6ST/JAK2/STAT3 Pathway in Vivo and in Vitro.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241261616. doi: 10.1177/15330338241261616. Technol Cancer Res Treat. 2024. PMID: 39051528 Free PMC article.
References
-
- Baxter, E.J. , Scott, L.M. , Campbell, P.J. , East, C. , Fourouclas, N. , Swanton, S. , Vassiliou, G.S. , Bench, A.J. , Boyd, E.M. , Curtin, N. , Scott, M.A. , Erber, W.N. , Green, A.R. , 2005. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 365, 1054–1061. - PubMed
-
- Bill, M.A. , Fuchs, J.R. , Li, C. , Yui, J. , Bakan, C. , Benson, D.M. , Schwartz, E.B. , Abdelhamid, D. , Lin, J. , Hoyt, D.G. , Fossey, S.L. , Young, G.S. , Carson, W.E. , Li, P.K. , Lesinski, G.B. , 2010. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol. Cancer. 9, 165 - PMC - PubMed
-
- Blaskovich, M.A. , Sun, J. , Cantor, A. , Turkson, J. , Jove, R. , Sebti, S.M. , 2003. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res.. 63, 1270–1279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

