Protective role for the disulfide isomerase PDIA3 in methamphetamine neurotoxicity
- PMID: 22715419
- PMCID: PMC3371042
- DOI: 10.1371/journal.pone.0038909
Protective role for the disulfide isomerase PDIA3 in methamphetamine neurotoxicity
Abstract
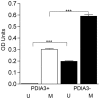
Methamphetamine abuse continues to be a worldwide problem, damaging the individual user as well as society. Only minimal information exists on molecular changes in the brain that result from methamphetamine administered in patterns typical of human abusers. In order to investigate such changes, we examined the effect of methamphetamine on the transcriptional profile in brains of monkeys. Gene expression profiling of caudate and hippocampus identified protein disulfide isomerase family member A3 (PDIA3) to be significantly up-regulated in the animals treated with methamphetamine as compared to saline treated control monkeys. Methamphetamine treatment of mice also increased striatal PDIA3 expression. Treatment of primary striatal neurons with methamphetamine revealed an up-regulation of PDIA3, showing a direct effect of methamphetamine on neurons to increase PDIA3. In vitro studies using a neuroblastoma cell line demonstrated that PDIA3 expression protects against methamphetamine-induced cell toxicity and methamphetamine-induced intracellular reactive oxygen species production, revealing a neuroprotective role for PDIA3. The current study implicates PDIA3 to be an important cellular neuroprotective mechanism against a toxic drug, and as a potential target for therapeutic investigations.
Conflict of interest statement
Figures

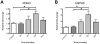


Similar articles
-
Protein disulfide-isomerase A3 significantly reduces ischemia-induced damage by reducing oxidative and endoplasmic reticulum stress.Neurochem Int. 2019 Jan;122:19-30. doi: 10.1016/j.neuint.2018.11.002. Epub 2018 Nov 3. Neurochem Int. 2019. PMID: 30399388
-
Drugs of abuse-induced hyperthermia, blood-brain barrier dysfunction and neurotoxicity: neuroprotective effects of a new antioxidant compound H-290/51.Curr Pharm Des. 2007;13(18):1903-23. doi: 10.2174/138161207780858375. Curr Pharm Des. 2007. PMID: 17584116 Review.
-
S-Nitrosylating protein disulphide isomerase mediates α-synuclein aggregation caused by methamphetamine exposure in PC12 cells.Toxicol Lett. 2014 Oct 1;230(1):19-27. doi: 10.1016/j.toxlet.2014.07.026. Epub 2014 Jul 30. Toxicol Lett. 2014. PMID: 25090657
-
Patterns of methamphetamine abuse and their consequences.J Addict Dis. 2002;21(1):21-34. doi: 10.1300/j069v21n01_03. J Addict Dis. 2002. PMID: 11831497 Review.
-
Protein Disulfide-Isomerase A3 Is a Robust Prognostic Biomarker for Cancers and Predicts the Immunotherapy Response Effectively.Front Immunol. 2022 Mar 25;13:837512. doi: 10.3389/fimmu.2022.837512. eCollection 2022. Front Immunol. 2022. PMID: 35401558 Free PMC article.
Cited by
-
Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings.Nutrients. 2021 Oct 20;13(11):3672. doi: 10.3390/nu13113672. Nutrients. 2021. PMID: 34835929 Free PMC article. Review.
-
[Effect of methamphetamine exposure on S-nitrosylation of protein disulphide isomerase in PC12 cells].Nan Fang Yi Ke Da Xue Xue Bao. 2017 Jan 20;37(1):93-96. doi: 10.3969/j.issn.1673-4254.2017.01.17. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 28109106 Free PMC article. Chinese.
-
Chronic SIV and morphine treatment increases heat shock protein 5 expression at the synapse.J Neurovirol. 2015 Oct;21(5):592-8. doi: 10.1007/s13365-015-0356-9. Epub 2015 Jun 3. J Neurovirol. 2015. PMID: 26037114 Free PMC article.
-
Stress protein expression in early phase spinal cord ischemia/reperfusion injury.Neural Regen Res. 2013 Aug 25;8(24):2225-35. doi: 10.3969/j.issn.1673-5374.2013.24.002. Neural Regen Res. 2013. PMID: 25206532 Free PMC article.
-
Integrated Systems Analysis of Mixed Neuroglial Cultures Proteome Post Oxycodone Exposure.Int J Mol Sci. 2021 Jun 15;22(12):6421. doi: 10.3390/ijms22126421. Int J Mol Sci. 2021. PMID: 34203972 Free PMC article.
References
-
- Harvey DC, Lacan G, Melegan WP. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res. 2000;133:358. - PubMed
-
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:270. - PubMed
-
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

