Lysine post-translational modifications of collagen
- PMID: 22708567
- PMCID: PMC3499978
- DOI: 10.1042/bse0520113
Lysine post-translational modifications of collagen
Abstract
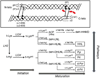
Type I collagen is the most abundant structural protein in vertebrates. It is a heterotrimeric molecule composed of two α1 chains and one α2 chain, forming a long uninterrupted triple helical structure with short non-triple helical telopeptides at both the N- and C-termini. During biosynthesis, collagen acquires a number of post-translational modifications, including lysine modifications, that are critical to the structure and biological functions of this protein. Lysine modifications of collagen are highly complicated sequential processes catalysed by several groups of enzymes leading to the final step of biosynthesis, covalent intermolecular cross-linking. In the cell, specific lysine residues are hydroxylated to form hydroxylysine. Then specific hydroxylysine residues located in the helical domain of the molecule are glycosylated by the addition of galactose or glucose-galactose. Outside the cell, lysine and hydroxylysine residues in the N- and C-telopeptides can be oxidatively deaminated to produce reactive aldehydes that undergo a series of non-enzymatic condensation reactions to form covalent intra- and inter-molecular cross-links. Owing to the recent advances in molecular and cellular biology, and analytical technologies, the biological significance and molecular mechanisms of these modifications have been gradually elucidated. This chapter provides an overview on these enzymatic lysine modifications and subsequent cross-linking.
Figures




Similar articles
-
Analysis of collagen and elastin cross-links.Methods Cell Biol. 2018;143:115-132. doi: 10.1016/bs.mcb.2017.08.006. Epub 2017 Nov 22. Methods Cell Biol. 2018. PMID: 29310773
-
Lysine Hydroxylation and Cross-Linking of Collagen.Methods Mol Biol. 2019;1934:309-324. doi: 10.1007/978-1-4939-9055-9_19. Methods Mol Biol. 2019. PMID: 31256387
-
Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER.J Biol Chem. 2021 Jan-Jun;296:100453. doi: 10.1016/j.jbc.2021.100453. Epub 2021 Feb 23. J Biol Chem. 2021. PMID: 33631195 Free PMC article.
-
Collagen hydroxylysine glycosylation: non-conventional substrates for atypical glycosyltransferase enzymes.Biochem Soc Trans. 2021 Apr 30;49(2):855-866. doi: 10.1042/BST20200767. Biochem Soc Trans. 2021. PMID: 33704379 Review.
-
Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance.Bone. 1998 Mar;22(3):181-7. doi: 10.1016/s8756-3282(97)00279-2. Bone. 1998. PMID: 9514209 Review.
Cited by
-
Wnt signaling and Loxl2 promote aggressive osteosarcoma.Cell Res. 2020 Oct;30(10):885-901. doi: 10.1038/s41422-020-0370-1. Epub 2020 Jul 20. Cell Res. 2020. PMID: 32686768 Free PMC article.
-
Post-translational hydroxylation by 2OG/Fe(II)-dependent oxygenases as a novel regulatory mechanism in bacteria.Front Microbiol. 2015 Jan 15;5:798. doi: 10.3389/fmicb.2014.00798. eCollection 2014. Front Microbiol. 2015. PMID: 25642226 Free PMC article. Review.
-
The application of collagen in the repair of peripheral nerve defect.Front Bioeng Biotechnol. 2022 Sep 23;10:973301. doi: 10.3389/fbioe.2022.973301. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36213073 Free PMC article. Review.
-
Seeking for Innovation with Magnetic Resonance Imaging Paramagnetic Contrast Agents: Relaxation Enhancement via Weak and Dynamic Electrostatic Interactions with Positively Charged Groups on Endogenous Macromolecules.J Am Chem Soc. 2024 Jan 10;146(1):134-144. doi: 10.1021/jacs.3c06275. Epub 2023 Dec 28. J Am Chem Soc. 2024. PMID: 38152996 Free PMC article.
-
Advances in Noninvasive Molecular Imaging Probes for Liver Fibrosis Diagnosis.Biomater Res. 2024 Jul 1;28:0042. doi: 10.34133/bmr.0042. eCollection 2024. Biomater Res. 2024. PMID: 38952717 Free PMC article. Review.
References
-
- Carter EM, Raggio CL. Genetic and orthopedic aspects of collagen disorders. Curr. Opin. Pediatr. 2009;21:46–54. - PubMed
-
- Hulmes DJS. Collagen diversity, synthesis and assembly. In: Fratzl P, editor. Collagen. London: Springer; 2008. pp. 15–47.
-
- Leitinger B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011;27:265–290. - PubMed
-
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell. 1997;1:13–23. - PubMed
-
- Bhadriraju K, Chung KH, Spurlin TA, Haynes RJ, Elliott JT, Plant AL. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials. 2009;30:6687–6694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

