Mitochondrial protein acetylation regulates metabolism
- PMID: 22708561
- PMCID: PMC3872051
- DOI: 10.1042/bse0520023
Mitochondrial protein acetylation regulates metabolism
Abstract
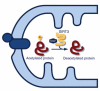
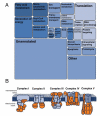
Changes in cellular nutrient availability or energy status induce global changes in mitochondrial protein acetylation. Over one-third of all proteins in the mitochondria are acetylated, of which the majority are involved in some aspect of energy metabolism. Mitochondrial protein acetylation is regulated by SIRT3 (sirtuin 3), a member of the sirtuin family of NAD+-dependent protein deacetylases that has recently been identified as a key modulator of energy homoeostasis. In the absence of SIRT3, mitochondrial proteins become hyperacetylated, have altered function, and contribute to mitochondrial dysfunction. This chapter presents a review of the functional impact of mitochondrial protein acetylation, and its regulation by SIRT3.
Figures
Similar articles
-
NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10.J Biol Chem. 2010 Mar 5;285(10):7417-29. doi: 10.1074/jbc.M109.053421. Epub 2009 Dec 30. J Biol Chem. 2010. PMID: 20042612 Free PMC article.
-
Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6601-6. doi: 10.1073/pnas.1302961110. Epub 2013 Apr 1. Proc Natl Acad Sci U S A. 2013. PMID: 23576753 Free PMC article.
-
Sirtuin 3 regulates mitochondrial protein acetylation and metabolism in tubular epithelial cells during renal fibrosis.Cell Death Dis. 2021 Sep 13;12(9):847. doi: 10.1038/s41419-021-04134-4. Cell Death Dis. 2021. PMID: 34518519 Free PMC article.
-
Mitochondrial sirtuins.Biochim Biophys Acta. 2010 Aug;1804(8):1645-51. doi: 10.1016/j.bbapap.2009.12.021. Epub 2010 Jan 7. Biochim Biophys Acta. 2010. PMID: 20060508 Review.
-
Sirtuin 3: Emerging therapeutic target for cardiovascular diseases.Free Radic Biol Med. 2022 Feb 20;180:63-74. doi: 10.1016/j.freeradbiomed.2022.01.005. Epub 2022 Jan 11. Free Radic Biol Med. 2022. PMID: 35031448 Review.
Cited by
-
A ketogenic diet impacts markers of mitochondrial mass in a tissue specific manner in aged mice.Aging (Albany NY). 2021 Mar 18;13(6):7914-7930. doi: 10.18632/aging.202834. Epub 2021 Mar 18. Aging (Albany NY). 2021. PMID: 33735837 Free PMC article.
-
Mitochondrial epigenetic modifications and nuclear-mitochondrial communication: A new dimension towards understanding and attenuating the pathogenesis in women with PCOS.Rev Endocr Metab Disord. 2023 Apr;24(2):317-326. doi: 10.1007/s11154-023-09789-2. Epub 2023 Jan 27. Rev Endocr Metab Disord. 2023. PMID: 36705802 Free PMC article. Review.
-
Mitochondrial Autophagy in Ischemic Aged Livers.Cells. 2022 Dec 16;11(24):4083. doi: 10.3390/cells11244083. Cells. 2022. PMID: 36552847 Free PMC article. Review.
-
Mitochondrial Function, Metabolic Regulation, and Human Disease Viewed through the Prism of Sirtuin 4 (SIRT4) Functions.J Proteome Res. 2019 May 3;18(5):1929-1938. doi: 10.1021/acs.jproteome.9b00086. Epub 2019 Apr 8. J Proteome Res. 2019. PMID: 30913880 Free PMC article. Review.
-
SIRT3 Mediates the Antioxidant Effect of Hydrogen Sulfide in Endothelial Cells.Antioxid Redox Signal. 2016 Feb 20;24(6):329-43. doi: 10.1089/ars.2015.6331. Epub 2015 Nov 10. Antioxid Redox Signal. 2016. PMID: 26422756 Free PMC article.
References
-
- Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. - PubMed
-
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell. 2006;23:607–618. - PubMed
-
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

